AbbVie (ABBV), J&J's Imbruvica Gets FDA Nod for cGVHD in Kids
AbbVie ABBV and partner J&J JNJ announced that Imbruvica, their blockbuster BTK inhibitor (BTKi) drug, has received label expansion approval from the FDA to treat chronic graft versus host disease (cGVHD) in pediatric patients.
The FDA granted approval to use Imbruvica in pediatric patients aged one year and older who have failed at least one line of systemic therapy. The drug is already approved for treating cGVHD in adults.
Please note that this is the 12th FDA approval secured by both AbbVie and J&J for Imbruvica. The approval also makes Imbruvica the first and currently the only approved treatment for children under 12 years of age suffering from cGVHD.
The FDA decision is based on data from the phase I/II iMAGINE study, which evaluated Imbruvica in pediatric and young adult patients aged one year to less than 22 years suffering from moderate or severe cGVHD. Data from the study showed that patients aged between 1 to 19 years treated with Imbruvica achieved an overall response rate (ORR) of 60% through week 25. The safety profile of the drug was consistent with those observed in adult patients.
While shares of AbbVie have gained 1.9% in the year so far, J&J’s shares have lost 3.2%. The industry has declined 0.6% year to date.

Image Source: Zacks Investment Research
cGVHD is a rare condition, which can occur in patients who have received peripheral blood or bone marrow stem cell transplantation due to treatments for blood cancer. cGVHD arises when the donated blood or stem cells launch an immune attack on the recipient’s body. This impacts major body organs of the recipient, including the skin and mouth. Presently, the current standard of treatment for cGVHD in the pediatric population is steroids.
Per J&J, the drug’s unique kinase profile gives it a clinical benefit for cGVHD. In fact, Imbruvica inhibits both Bruton's tyrosine kinase (BTK) and interleukin-2-inducible T-cell kinase (ITK) proteins.
Apart from cGVHD, Imbruvica is approved for multiple indications, including five hematologic cancers. These include chronic lymphocytic leukemia (CLL)/small lymphocytic lymphoma (SLL), mantle cell lymphoma,Waldenström’s macroglobulinemia and marginal zone lymphoma in various settings and patient population
AbbVie has developed Imbruvica in collaboration with J&J. Per the terms of the agreement, AbbVie and J&J jointly market Imbruvica in the United States, while J&J holds exclusive rights for marketing the drug outside the country. In the second quarter of 2022, AbbVie recorded $1.15 billion as worldwide net revenues from Imbruvica sales. This $1.15 billion figure includes $283 million recorded by AbbVie as a share of profit from the international sales of the J&J-partnered drug.
AbbVie Inc. Price
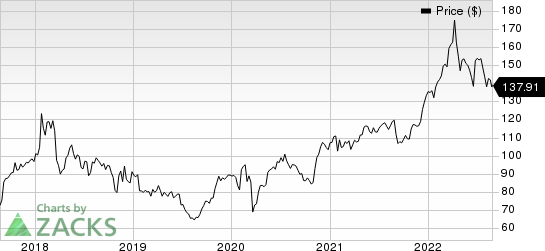
AbbVie Inc. price | AbbVie Inc. Quote
Johnson & Johnson Price
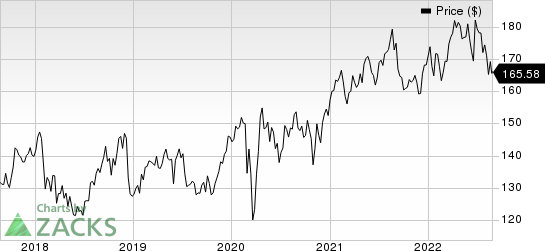
Johnson & Johnson price | Johnson & Johnson Quote
Zacks Rank & Stocks to Consider
AbbVie and J&J both currently carry a Zacks Rank #3 (Hold). Some better-ranked stocks in the overall healthcare sector include Assertio ASRT and Morphic MORF. While Morphic sports a Zacks Rank #1 (Strong Buy) at present, Assertio carries a Zacks Rank #2 (Buy). You can see the complete list of today’s Zacks #1 Rank stocks here.
In the past 30 days, estimates for Morphic’s 2022 loss per share have narrowed from $3.38 to $1.75. Loss estimates for 2023 have narrowed from $3.91 to $3.77 during the same period. Shares of Morphic have lost 33.7% in the year-to-date period.
Earnings of Morphic beat estimates in three of the last four quarters and missed the mark just once, witnessing a surprise of 48.29%, on average. In the last reported quarter, MORF delivered an earnings surprise of 183.95%.
In the past 30 days, estimates for Assertio’s 2022 earnings per share have risen from 40 cents to 49 cents. Earnings estimates for 2023 have risen from 29 cents to 30 cents during the same period. Shares of Assertio have risen 32.6% in the year-to-date period.
Earnings of Assertio beat estimates in three of the last four quarters and missed the mark just once, witnessing a surprise of 126.39%, on average. In the last reported quarter, ASRT delivered an earnings surprise of 100.00%.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Johnson & Johnson (JNJ) : Free Stock Analysis Report
AbbVie Inc. (ABBV) : Free Stock Analysis Report
Assertio Holdings, Inc. (ASRT) : Free Stock Analysis Report
Morphic Holding, Inc. (MORF) : Free Stock Analysis Report
To read this article on Zacks.com click here.
Zacks Investment Research

 Yahoo Finance
Yahoo Finance 