Biogen (BIIB) & Eisai's Lecanemab Secures EMA's MAA Acceptance
Biogen Inc. BIIB and Japanese partner Eisai,announced that the European Medicine Agency (EMA) accepted its marketing authorization application (MAA) for standard review for lecanemab to treat early-stage Alzheimer’s disease (mild cognitive impairment due to Alzheimer’s disease [AD] and mild AD dementia), having confirmed amyloid pathology.
Earlier this January, lecanemab was granted accelerated approval in the United States for the treatment of AD by the FDA. The drug will be marketed under the brand name Leqembi. Its label mentions that treatment with the drug should be initiated in patients with mild cognitive impairment or mild dementia stage of disease, the population in which treatment was initiated in clinical studies. Based on data from this study, Eisai also filed a supplemental biologics license application (sBLA) to the FDA to get traditional approval for Leqembi. This sBLA submission wasbased on data from the phase III Clarity AD confirmatory study.
Lecanemab is a monoclonal antibody that selectively binds and eliminates amyloid-beta (Aβ) protofibrils which cause neurotoxicity in AD. The accelerated approval was based on data from a phase II study (Study 201), which showed that treatment with lecanemab reduced the accumulation of Aβ plaque in the brain.
The Clarity AD study, a large phase III confirmatory study of lecanemab met its primary endpoint as well as all key secondary endpoints, the results being statistically significant.
Biogen collaborated with Eisai to co-develop and co-commercialize AD treatments since 2014. Per the terms of the agreement, Eisai leads lecanemab development and regulatory submissions worldwide and Biogen has co-promotion rights, while Eisai enjoys final decision-making authority.
In the past year, shares of Biogen have increased 29.6% against the industry’s decline of 6.4%.
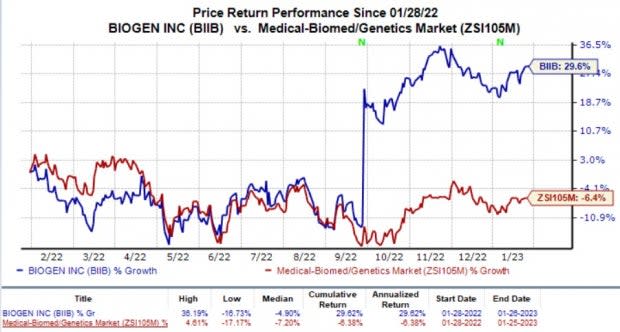
Image Source: Zacks Investment Research
Lecanemab’s success is however subject to stiff competition from other players in the market. Recently, Eli Lilly LLY announced that the FDA issued a complete response letter (CRL) to its regulatory filing seeking accelerated approval of a new Alzheimer’s drug, donanemab.
Per the FDA, the CRL was issued as the study supporting the regulatory filing had limited information on patients who received a minimum of 12 months of continued treatment on donanemab. The CRL specifically requested Lilly to provide data from at least 100 patients with at least 12 months of exposure to donanemab. Based on the responses received in the CRL, Lilly will work closely with the FDA to evaluate the fastest pathway to resolve the FDA’s queries. Per management, this will not impact the financial guidance for 2023 issued by the company last month.
AC Immune SA ACIU announced encouraging and promising initial safety and immunogenicity findings for its candidate, ACI-24.060, from the phase Ib/II ABATE study. Preliminary results from the first cohort of patients showed that a low dose of the candidate generated a positive response in week 6, that is just 2 weeks after the administration of the second injection. Based on the satisfactory results, AC Immune has begun testing higher doses in patients with AD in the ABATE study. AC Immune is set to expand the scope of the ABATE study by screening individuals with Down syndrome for the second part of the study.
Zacks Rank and Stock to Consider
Biogen presently has a Zacks Rank #3 (Hold).
A better-ranked stock in the same industry is Adicet Bio, Inc. ACET, carrying a Zacks Rank #2 (Buy). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
In the past 90 days, the estimate for Adicet Bio’s 2022 loss per share has narrowed from $1.63 to $1.54. During the same period, the loss estimate per share for 2023 has narrowed from $2.25 to $2.09. In the past year, the shares of Adicet have decreased by 23.9%.
ACET’s earnings witnessed an average earnings surprise of 54.24%, beating two out of the four surprise estimates in the trailing four quarters.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Biogen Inc. (BIIB) : Free Stock Analysis Report
Eli Lilly and Company (LLY) : Free Stock Analysis Report
Adicet Bio, Inc. (ACET) : Free Stock Analysis Report
AC Immune (ACIU) : Free Stock Analysis Report

 Yahoo Finance
Yahoo Finance 