Biotech Stock Roundup: ALT Obesity Data, GILD Exercises Option, INCY Drug Approval
The biotech sector has been in focus in the past week with key pipeline and regulatory updates. Among these, Incyte’s INCY skin cancer drug obtained FDA approval. Altimmune ALT declined on mixed obesity data.
Recap of the Week’s Most Important Stories:
Altimmune Down on Obesity Data: Clinical-stage biopharmaceutical company Altimmune, announced top-line results from the interim analysis of 160 subjects in its 48-week phase II MOMENTUM obesity study of pemvidutide at week 24 along with the results of the 12-week phase Ib safety trial of pemvidutide in subjects with obesity or overweight and type 2 diabetes.
Data showed subjects receiving pemvidutide achieved mean weight losses of 7.3%, 9.4% and 10.7% at the 1.2 mg, 1.8 mg, and 2.4 mg doses, respectively, with the placebo group experiencing a mean weight loss of 1.0% at week 24. Of the subject population comprising subjects with baseline body weight less than or equal to 115 kg, 75% witnessed a mean weight loss of 8.2%, 10.6%, 11.9% and 0.8% at the 1.2 mg, 1.8 mg, 2.4 mg and placebo groups, respectively. Approximately 50% of subjects achieved 10% or more weight loss and about 20% of subjects achieved 15% or more weight loss at week 24 at the 1.8 mg and 2.4 mg doses.
However, investors were most likely disappointed with adverse events (AEs), which mainly comprised upper gastrointestinal (GI) events of nausea and vomiting. One subject (2.4%) experienced a serious adverse event of nausea and vomiting, requiring rehydration at the 2.4 mg dose. Treatment discontinuation rates were 28.2% in subjects receiving placebo and 24.0% in subjects receiving pemvidutide.
Results from the 12-week phase Ib study to treat obesity or overweight and type II diabetes showed average weight losses of 4.4%, 6.1% and 7.7% at the 1.2 mg, 1.8 mg and 2.4 mg doses of pemvidutide, respectively, with the placebo group experiencing a mean weight gain of 0.8%
Biogen’s Update on Tofersen NDA: Biogen BIIB announced the outcome of the FDA’s Peripheral and Central Nervous System Drugs Advisory Committee meeting on pipeline candidate. The candidate is being evaluated for the treatment of superoxide dismutase 1 (SOD1) amyotrophic lateral sclerosis (ALS), an ultra-rare, progressive and fatal neurodegenerative disease that results in the loss of motor neurons in the brain and the spinal cord that are responsible for controlling voluntary muscle movement. Biogen licensed tofersen from Ionis Pharmaceuticals, Inc. under a collaborative development and license agreement.
The committee voted unanimously 9:0 that reductions of neurofilament, a marker of neurodegeneration, observed in tofersen-treated patients in the clinical studies, are reasonably likely to predict the clinical benefit of tofersen for treating SOD1-ALS. However, the opinion was mostly negative on the question of data from the placebo-controlled study and available long-term extension study results, with additional supporting results from the effects on relevant biomarkers providing substantial evidence of the effectiveness of tofersen in the treatment of patients with SOD1-ALS. The committee voted 3:5 and one person abstained from voting on consideration of a potential traditional approval. Additionally, the committee discussed both topics and reached a consensus that the benefit-risk profile was favorable based on the review of the totality of data for tofersen in people with SOD1-ALS.
The NDA for tofersen for the treatment of SOD1-ALS was submitted to the FDA for consideration under accelerated approval. The agency is continuing its review of tofersen with a target action date of Apr 25, 2023.
Gilead Exercises Option for Targeted Protein Degrader: Gilead Sciences, Inc. GILD announced that it has exercised its option to exclusively license Nurix Therapeutics’ investigational targeted protein degrader molecule NX 0479. Both companies announced a global strategic collaboration in 2019 to discover, develop and commercialize a pipeline of innovative targeted protein degradation drugs for cancer patients and other challenging diseases.
Bivalent degrader NX 0479, designated as GS-6791, is the first development candidate resulting from this collaboration. The GS-6791 is a potent, selective, oral IRAK4 degrader that targets both the scaffold and kinase functions of the IRAK4 protein kinase to block inflammatory responses downstream of toll-like receptors (TLR) and the pro-inflammatory IL1 cytokine family of receptors (IL1Rs). The IRAK4 degradation has potential applications in the treatment of rheumatoid arthritis and other inflammatory diseases.
Per the terms for the NX-0479 option that Gilead is exercising, Nurix will receive an option exercise payment of $20 million and could receive up to an additional $425 million in milestone payments, as well as up to low double-digit tiered royalties on product net sales. Shares of Nurix were up as investors seemed pleased about the cash inflow from the option exercise.
Karuna’s Schizophrenia Study Data: Karuna Therapeutics, Inc. KRTX announced that its late-stage EMERGENT-3 stuyd evaluating the efficacy, safety and tolerability of its lead investigational therapy, KarXT (xanomeline-trospium) in adults with schizophrenia met its primary endpoint. KarXT demonstrated a statistically significant and clinically meaningful 8.4-point reduction in Positive and Negative Syndrome Scale (PANSS) total score compared to placebo. Treatment with KarXT demonstrated reductions in both positive symptoms (like hallucinations or delusions) and negative symptoms (like, difficulty enjoying life or withdrawal from others) of schizophrenia, the secondary endpoints of the study. Although KarXT demonstrated a clinically meaningful and statistically significant 3.5-point reduction in PANSS positive subscale compared to placebo at week five (-7.1 KarXT vs. -3.6 placebo; p<0.0001), it did not meet the threshold for statistical significance at week five.
Incyte’s Drug Approval: Incyte INCY announced that the FDA has granted accelerated approval to a biologics license application (BLA) for intravenous PD-1 inhibitor retifanlimab-dlwr. under the brand name Zynyz. The BLA was seeking approval to treat adults with metastatic or recurrent locally advanced merkel cell carcinoma (MCC).
The accelerated approval was granted based on the tumor response rate and duration of response (DOR). Continued approval of Zynyz for this indication may be contingent on verification and description of clinical benefit in confirmatory trials.
MCC, a rare and aggressive type of skin cancer, most often appears as a single, painless, reddish-purple skin nodule on the head, neck and arms in skin exposed to sunlight.
The FDA approval was based on data from the POD1UM-201 trial, an open-label, multiregional, single-arm study that evaluated Zynyz in adults with metastatic or recurrent locally advanced MCC who had not received prior systemic therapy for their advanced disease.
Performance
The Nasdaq Biotechnology Index has lost 2.22% in the past five trading sessions. Among the biotech giants, Glaxo has gained 3.93% during the period. Over the past six months, shares of Biogen have soared 30.21%. (See the last biotech stock roundup here: Biotech Stock Roundup: ACADs Drug Approval, SGEN to be Acquired by PFE & More)
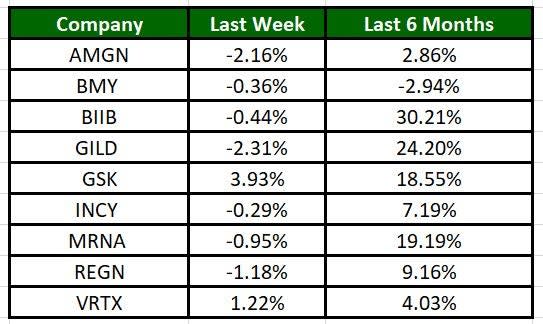
Image Source: Zacks Investment Research
What's Next in Biotech?
Stay tuned for other updates.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Biogen Inc. (BIIB) : Free Stock Analysis Report
Gilead Sciences, Inc. (GILD) : Free Stock Analysis Report
Incyte Corporation (INCY) : Free Stock Analysis Report
Altimmune, Inc. (ALT) : Free Stock Analysis Report
Karuna Therapeutics, Inc. (KRTX) : Free Stock Analysis Report

 Yahoo Finance
Yahoo Finance 