Biotech Stock Roundup: ICPT, KALV Down on Study Data, VIR Up on Contract Win & More
The biotech sector was in focus this past week with important regulatory and pipeline updates on key candidates.
Recap of the Week’s Most Important Stories:
Intercept Down on Study Failure: Shares of Intercept Pharmaceuticals, Inc ICPT were down after the company announced that a phase III study, REVERSE, evaluating the safety and efficacy of OCA in patients with compensated cirrhosis due to nonalcoholic steatohepatitis (NASH), did not meet its primary endpoint of a ≥ 1-stage histological improvement in fibrosis with no worsening of NASH following up to 18 months of therapy.
The study is one of Intercept’s two-phase III studies evaluating different populations in NASH. Results showed of the 919 randomized subjects with compensated cirrhosis due to NASH, 11.1% of subjects who were randomized to receive once-daily oral OCA 10 mg and 11.9% of subjects who were randomized to receive OCA 10 mg titrated to 25 mg (OCA 10-to-25 mg) after three months achieved a ≥1-stage improvement in fibrosis with no worsening of NASH after up to 18 months of treatment compared with 9.9% of subjects who received placebo.
Nevertheless, Intercept’s planned new drug application (NDA) for its lead indication of liver fibrosis due to NASH will be supported by positive phase III data from the REGENERATE study and not by the REVERSE study. Hence, the failure of the REVERSE study will not affect the planned NDA for the abovementioned indication. Intercept stated that it is on track to resubmit its NDA in liver fibrosis due to NASH by the end of 2022.
Intercept has a Zacks Rank #3 (Hold) currently. You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Vir Gains on Government Contract: Shares of Vir Biotechnology, Inc. VIR gained after the company was awarded a multi-year contract with the potential for up to $1 billion to advance the development of a full portfolio of innovative solutions to address influenza and potentially other infectious disease threats. The contract was awarded by the Biomedical Advanced Research and Development Authority (“BARDA”), part of the U.S. Department of Health and Human Services’ Administration for Strategic Preparedness and Response (ASPR).
Vir will receive an initial investment of approximately $55 million from BARDA to support the development of VIR-2482, an investigational prophylactic monoclonal antibody for seasonal and pandemic influenza viruses. This includes a phase II pre-exposure prophylaxis study expected to begin in the second half of this year with initial data anticipated in mid-2023. candidates.
Exelixis Expands Collaboration With BMY: Exelixis EXEL announced that it has expanded its collaboration and supply agreement with Bristol-Myers Squibb Company BMY to evaluate XL092 in combination with an additional immune checkpoint inhibitor. The new agreement will include the use of the fixed-dose combination of Opdivo (nivolumab) and relatlimab in the ongoing STELLAR-002 study, which is evaluating XL092 in combination with multiple immune checkpoint inhibitors in advanced solid tumors.
Enrollment and dosing in the dose-escalation portion of STELLAR-002 are ongoing. The dose-escalation stage will determine the recommended dose in patients with advanced solid tumors for each of the combination therapy regimens, including XL092 and nivolumab, XL092, nivolumab and ipilimumab, and XL092 and the fixed-dose combination of nivolumab and relatlimab. In 2021, Exelixis announced an agreement with BMY to evaluate the safety, tolerability and efficacy of XL092, in combination with nivolumab, nivolumab and Yervoy (ipilimumab) and nivolumab and bempegaldesleukin.
KalVista Crashes on Study Update: Shares of clinical-stage pharmaceutical company, KalVista Pharmaceuticals, Inc. KALV plunged significantly after the company announced that it has terminated the phase II KOMPLETE study for KVD824 for the prevention of attacks in people with hereditary angioedema (HAE). The decision to terminate was based on the observation of liver enzyme (ALT/AST) elevations in multiple patients in all treatment groups of the trial. None of the patients had a concomitant elevation of bilirubin levels and all were asymptomatic. The company concluded that the emerging safety profile of the current formulation will not meet its requirements for a best-in-class oral prophylactic therapy. Consequently, KalVista will now focus on advancing sebetralstat through the ongoing phase III program and toward a planned 2024 NDA filing, as well as on its emerging oral Factor XIIa inhibitor program as a potential once-daily prophylactic therapy for people with HAE.
Performance
The Nasdaq Biotechnology Index has gained 2.38% in the past five trading sessions. Among the biotech giants, Regeneron has gained 5.71% during the period. Over the past six months, shares of Biogen have soared 23.63%. (See the last biotech stock roundup here: Biotech Stock Roundup: BIIBs AD Drug Data & REGN, IONS, VRTXs Updates)
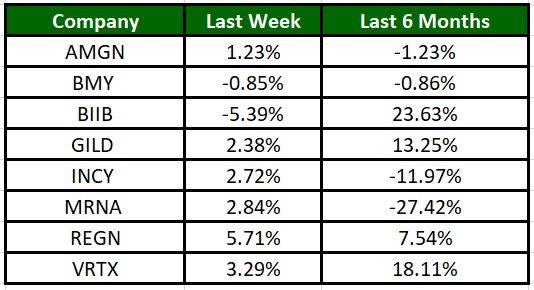
Image Source: Zacks Investment Research
What's Next in Biotech?
Stay tuned for other updates.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Bristol Myers Squibb Company (BMY) : Free Stock Analysis Report
Exelixis, Inc. (EXEL) : Free Stock Analysis Report
Intercept Pharmaceuticals, Inc. (ICPT) : Free Stock Analysis Report
KalVista Pharmaceuticals, Inc. (KALV) : Free Stock Analysis Report
Vir Biotechnology, Inc. (VIR) : Free Stock Analysis Report
To read this article on Zacks.com click here.
Zacks Investment Research

 Yahoo Finance
Yahoo Finance 