bluebird's (BLUE) Gene Therapy Gets FDA Nod for CALD
bluebird bio BLUE recently received accelerated FDA approval for Syskona (elivaldogene autotemcel), also known as eli-cel, as a treatment for patients less than 18 years of age with early, active cerebral adrenoleukodystrophy (CALD). Upon approval, bluebird also received a rare pediatric priority review voucher for Syskona.
The decision is in line with the recommendation of FDA’s Cellular, Tissue, and Gene Therapies Advisory Committee (CTGTAC). The committee voted unanimously (15-0) in favor of eli-cel in June 2022, stating that the benefits of eli-cel gene therapy outweigh the risks for treating any sub-population of children with early active CALD.
bluebird submitted a biologics license application (BLA) to the FDA for eli-cel as a treatment for patients less than 18 years of age with early CALD, which was accepted for priority review by the FDA in December 2021. Earlier this year, the FDA extended the review period for the BLA for Syskona (eli-cel) to review additional clinical information submitted by bluebird in response to the FDA request.
CALD is a rare progressive degenerative disease primarily affecting young males, causing a severe neurological decline. The complications include six major functional disabilities (MFDs) such as communication loss, cortical blindness, the requirement for tube feeding, partial or complete loss of voluntary movement and total incontinence. About 50% of CALD patients who do not receive treatment die within five years after the onset of its symptoms.
The accelerated approval for Syskona is based on patients who witnessed 24-month MFD-free survival. A post-hoc enrichment analysis was conducted in symptomatic CALD patients to assess MFD-free survival from the onset of symptoms in patients treated with Syskona versus untreated patients. The test exhibited an estimated 72% likelihood of MFD-free survival at 24 months from onset of symptoms in Syskona-treated patients compared with untreated patients who only had an estimated 43% likelihood of MFD-free survival.
Continued approval of Syskona for CALD may be dependent upon verification and description of clinical benefit in a confirmatory study.
Thus, as a condition of Syskona’s accelerated approval, bluebird will provide confirmatory long-term clinical data to the FDA from the ongoing long-term follow-up study (LTF-304), which follows patients treated in clinical trials for 15 years, and from commercially treated patients.
We remind investors that in August 2021, FDA had placed a clinical hold on bluebird’s eli-cel clinical program. The hold was put because of reported Suspected Unexpected Serious Adverse Reactions of myelodysplastic syndrome likely mediated by Lenti-D lentiviral vector insertion. In the meantime, all patients who received eli-cel in the clinical program continue to be closely monitored, per study protocols.
bluebird confirmed that the clinical hold on the eli-cel clinical development program was lifted on Sep 15, 2022, before the completion of FDA’s review of the BLA for Syskona.
The stock has fallen 36.6% in the year-to-date period compared with the industry’s decline of 22.5%.
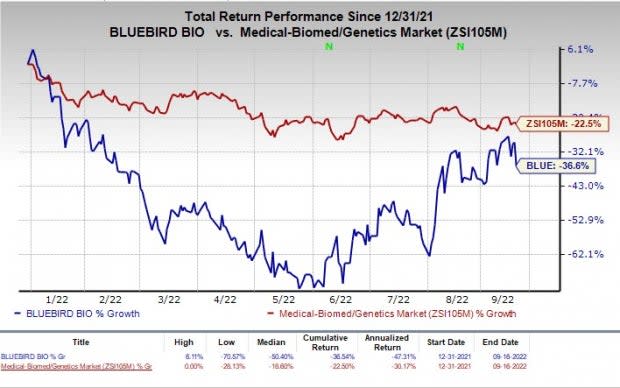
Image Source: Zacks Investment Research
With the approval in place, bluebird expects to commercially launch Syskona in the United States by the end of 2022 to enable patient access to the gene therapy as soon as possible.
The gene therapy, which represents a potential cure for CALD, will be available at a wholesale acquisition cost set by the company of $3 million per patient. The price represents the curative benefit of the therapy.
Syskona’s approval is bluebird’s second in two months. In August, FDA approved bluebird’s Zynteglo (betibeglogene autotemcel), as one-time gene therapy for treating beta-thalassemia, in adult and pediatric patients who require regular red blood cell (RBC) transfusions.
bluebird bio, Inc. Price
bluebird bio, Inc. price | bluebird bio, Inc. Quote
Zacks Rank and Stocks to Consider
bluebird currently has a Zacks Rank #3 (Hold).
Some better-ranked stocks in the same sector include Sesen Bio SESN, carrying a Zacks Rank #1 (Strong Buy) and Aridis Pharmaceuticals ARDS and Sutro Biopharma STRO, each carrying a Zacks Rank #2 (Buy). You can see the complete list of today’s Zacks #1 (Strong Buy) Rank stocks here.
Sesen Bio’s loss per share estimates for 2022 widened from 31 cents to 43 cents in the past 30 days. The same for 2023 has narrowed from 10 cents to a cent in the same time frame.
Earnings of Sesen Bio missed estimates in all of the trailing four quarters. The average negative earnings surprise for SESN is 89.49%.
Aridis Pharmaceuticals' loss estimates for 2022 have narrowed to 23 cents from a loss of 30 centys in the past 30 days. The loss estimates for 2023 also narrowed down from 58 cents per share to 53 cents per share in the same time frame.
ARDS surpassed earnings in three of the trailing four quarters, missing the same in one. The average negative earnings surprise for Aridis is 238.54%.
Sutro Biopharma’s loss per share estimates for 2022 remained steady at $2.23 in the past 30 days. The loss per share for 2023 has improved from $2.90 to $2.81 in the same time frame.
Earnings of Sutro Biopharma beat estimates in all of the trailing four quarters. The average negative earnings surprise for STRO is 1.13%.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
bluebird bio, Inc. (BLUE) : Free Stock Analysis Report
SESEN BIO, INC. (SESN) : Free Stock Analysis Report
Aridis Pharmaceuticals (ARDS) : Free Stock Analysis Report
Sutro Biopharma, Inc. (STRO) : Free Stock Analysis Report
To read this article on Zacks.com click here.
Zacks Investment Research

 Yahoo Finance
Yahoo Finance 