Catalent (CTLT) to Advance Oral Drug Development With New Launch
Catalent, Inc. CTLT recently launched a new Xpress Pharmaceutics service, which aims to further the development of oral drugs through Phase 1 clinical studies. This approach can lower the cost and time needed to complete first-in-human trials by combining formulation development, on-demand clinical manufacturing, regulatory support and clinical testing.
Formulation development experts at Catalent’s facilities situated in Nottingham, U.K., and Beinheim, France, will deliver the Xpress Pharmaceutics service, further backed by the company’s global Regulatory Affairs team. Catalent has a pre-qualified clinical research organization partner to support expedited timelines, allowing for a smooth transition from manufacturing to clinical testing.
The Xpress Pharmaceutics launch will likely bolster Catalent’s Oral and Specialty Delivery business.
Strategic Benefits of the Launch
The Xpress Pharmaceutics launch enables Catalent to collaborate with innovators and clinical research organizations and deliver formulated clinical trial material with the requisite stability data for dosing patients at the clinical site under adaptive study protocols. It is worth noting that this method is a speedier alternative to the traditional clinical development model. It also offers the added benefit of flexible dose and/or formulation composition adjustment during a clinical trial based on real-time clinical data.
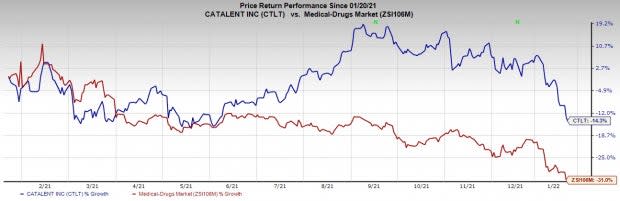
Image Source: Zacks Investment Research
Per management at Catalent, the new Xpress Pharmaceutics service integrates the company’s core expertise in drug formulation with manufacturing, analytics and regulatory expertise to develop better drugs for use in adaptive trials. The new service can halve the time to complete studies by focusing on critical areas for rapid progression to and through Phase 1 trials.
Industry Prospects
Per a report published in Grand View Research, the global clinical trials market will witness a CAGR of 5.7% from 2021 to 2028. The key factors contributing to market growth are the globalization of clinical trials, technological advancements, rising prevalence of chronic disease, and the increasing need for personalized medicines and orphan drugs.
Given the market prospects, the launch of Catalent’s Xpress Pharmaceutics service seems well-timed.
Notable Developments
In January 2022, Catalent expanded cold chain packaging capabilities at its 200,000-square-foot Philadelphia facility to meet the growing demand for the distribution of biologic drugs and advanced cell and gene therapies. The company is currently working to increase the packaging area at the Philadelphia facility by about 20,000 square feet, adding seven additional temperature-controlled processing suites. In addition, more ambient space has been added to provide packing operations and material storage services.
In December 2021, the technology transfer for BrainStorm Cell Therapeutics Inc.’s (BCLI) NurOwn manufacturing at Catalent’s facility was finalized. NurOwn, a BrainStorm autologous cellular therapy, is intended to treat amyotrophic lateral sclerosis, progressive multiple sclerosis and other neurodegenerative diseases. The manufacturing for NurOwn will take place in Houston, Texas, at Catalent’s world-class 32,000 square-foot cell therapy manufacturing facility.
Share Price Performance
The stock has declined 14.3% versus the industry’s 31% fall over the past year.
Zacks Rank and Other Key Picks
Currently, Catalent carries a Zacks Rank #2 (Buy).
Other top-ranked stocks in the broader medical space include Baxter International Inc. BAX, Hologic, Inc. HOLX and Cerner Corporation CERN.
Baxter, currently carrying a Zacks Rank #1 (Strong Buy), has a long-term earnings growth rate of 9.5%. Baxter’s earnings surpassed estimates in the trailing four quarters, delivering a surprise of 10.2%, on average. You can see the complete list of today’s Zacks #1 Rank stocks here.
Baxter has outperformed the industry over the past year. BAX has gained 7.6% against a 12.8% decline of the industry in the said period.
Hologic, carrying a Zacks Rank #1 at present, has a long-term earnings growth rate of 7.4%. The company surpassed earnings estimates in three of the trailing four quarters and missed another occasion, delivering an average surprise of 29.2%.
Hologic has declined 8.8% compared with the industry’s 8.1% drop over the past year.
Cerner carrying a Zacks Rank #2 at present, has a long-term earnings growth rate of 12.8%. Cerner’s earnings surpassed estimates in three of the trailing four quarters and met estimates on another occasion, delivering an average surprise of 3.2%.
Cerner has gained 15.1% against the industry’s 55.5% drop over the past year.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Cerner Corporation (CERN) : Free Stock Analysis Report
Baxter International Inc. (BAX) : Free Stock Analysis Report
Hologic, Inc. (HOLX) : Free Stock Analysis Report
Catalent, Inc. (CTLT) : Free Stock Analysis Report
To read this article on Zacks.com click here.
Zacks Investment Research

 Yahoo Finance
Yahoo Finance 