Dare's (DARE) Xaciato Gets FDA Nod for Bacterial Vaginosis
Daré Bioscience, Inc. DARE has announced that the FDA approved Xaciato (clindamycin phosphate vaginal gel, 2%) for the treatment of bacterial vaginosis in females 12 years of age and older.
Xaciato was formerly known as DARE-BV1.
We note that Xaciato received both Qualified Infectious Disease Product (QIDP) and Fast Track designations from the FDA for the treatment of bacterial vaginosis. Due to the QIDP designation, Xaciato is expected to receive a five-year extension of the three years of market exclusivity available to the product based on the submission of new clinical data essential to its approval.
Daré Bioscience is currently looking for partners to market Xaciato in the United States. A commercial launch is expected in 2022.
The approval will boost the growth prospects of the company as this is the first approved product for Daré Bioscience.
The new drug application was supported by positive results from the DARE-BVFREE study, which evaluated Xaciato in women diagnosed with bacterial vaginosis.
Bacterial vaginosis, the most common cause of vaginitis worldwide, is estimated to affect approximately 21 million women in the United States.
The stock has rallied 58.3% so far this year against the industry’s 19.6% decline.
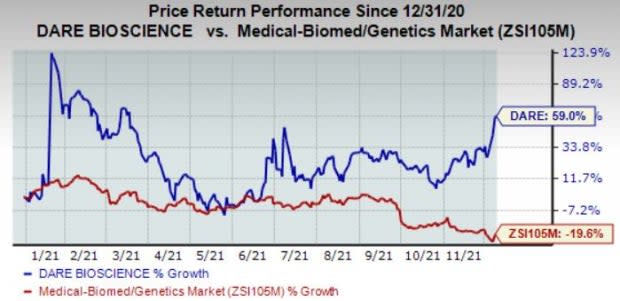
Image Source: Zacks Investment Research
Daré Bioscience’s product portfolio also includes potential first-in-category candidates in clinical development - Ovaprene, a novel, hormone-free monthly contraceptive for which it has a license agreement with Bayer BAYRY. Further, it comprises sildenafil cream, 3.6%, a proprietary cream formulation of sildenafil for topical administration to the vulva and vagina for treatment of female sexual arousal disorder.
In January 2020, the company entered into a license agreement with Bayer regarding the further development and commercialization of Ovaprene in the United States. The company received a $1.0 million upfront license fee payment from Bayer upon execution of the agreement.
The company continues to enroll patients in its phase IIb RESPOND study of sildenafil cream, 3.6%.
Zacks Rank & Stocks to Consider
Daré Bioscience currently carries a Zacks Rank #3 (Hold). A couple of better-ranked stocks in the healthcare sector are Sarepta Therapeutics, Inc. SRPT and Viking Therapeutics VKTX, both carrying a Zacks Rank #2 (Buy) at present. You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Estimates for Sarepta have narrowed from a loss per share of $6.95 to $4.99 for 2021 and from $4.83 to $3.61 for 2022 in the past 30 days. SRPT delivered an earnings surprise of 11.06%, on average, in the last four quarters.
Estimates for Viking Therapeutics have narrowed to a loss per share of $3.16 from $3.55 for 2021 and to $3.47 from $3.63 for 2022 in the past 30 days. VKTX delivered an earnings surprise of 2.06%, on average, in the last four quarters.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Bayer Aktiengesellschaft (BAYRY) : Free Stock Analysis Report
Sarepta Therapeutics, Inc. (SRPT) : Free Stock Analysis Report
Viking Therapeutics, Inc. (VKTX) : Free Stock Analysis Report
Dare Bioscience, Inc. (DARE) : Free Stock Analysis Report
To read this article on Zacks.com click here.
Zacks Investment Research

 Yahoo Finance
Yahoo Finance 