Esperion Therapeutics (ESPR) Down 24% in a Week: Here's Why
In the past week, the share price of Esperion Therapeutics ESPR was down 24% compared with the industry’s 2.3% fall.
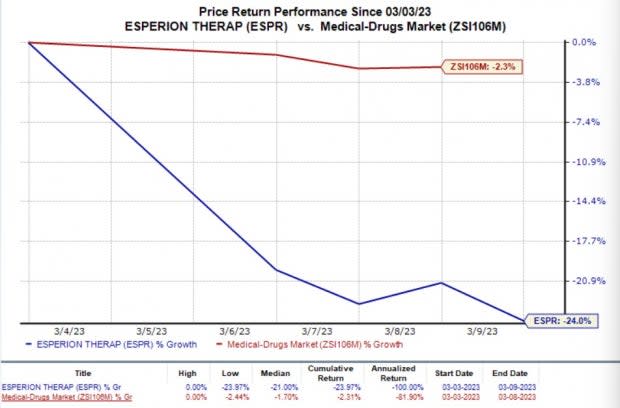
Image Source: Zacks Investment Research
The primary reason behind the drastic fall in share price was the full data presentation from the company’s cardiovascular outcomes trial (CVOT) study — CLEAR —evaluating the impact of its cholesterol medicine, Nexletol on reducing the risk of major cardiovascular (CV) events in statin-intolerant patients with or at high risk for, CV disease, on Mar 4, 2023, which failed to meet investors’ expectations.
Esperion has two approved drugs — Nexletol (bempedoic acid [180mg] and Nexlizet (bempedoic acid [180mg]/ ezetimibe [10mg] combination pill) — in its portfolio, which were approved in February 2020 in the United States. The drugs are approved as an adjunct to diet and maximally tolerated statin therapy for the treatment of adults with heterozygous familial hypercholesterolemia (HeFH) or established atherosclerotic cardiovascular disease (ASCVD), who require additional lowering of LDL-C in the United States. The CLEAR outcomes study is a phase III cardiovascular outcomes study designed to evaluate whether treatment with bempedoic acid reduces the risk of CV events in patients with or at high risk for CV disease with documented statin intolerance (inability to tolerate two or more statins, one at a low dose) and elevated LDL-cholesterol levels.
In December 2022, management announced that the CLEAR study achieved its primary endpoint. Full data from the study, presented recently, showed that Nexletol significantly reduced the risk of major adverse cardiovascular events (MACE-4) and (MACE-3) by 13% and 15%, respectively, compared to placebo. It also reduced the risk of heart attack and coronary revascularization by 23% and 19%, respectively, as compared with placebo.
However, the CLEAR outcomes study results failed to impress the investors. The company is planning to submit regulatory filings to the FDA and the European Medicine Agency in the first half of 2023 to seek approval for inclusion of cardiovascular risk reduction data on the drug’s label based on the results from the CLEAR Outcomes study. The acceptance of the same for review in Europe would make the company entitled to $300 million under partner milestones payment. For U.S. expansion of the label, the company is entitled to partner milestone payment of up to $140 million. Esperion anticipates a decision from the aforementioned regulatory bodies to expand the CV risk-reduction label in the first half of 2024, which management claims, will double the addressable treatment population for Nexletol and Nexlizet.
Esperion investors still have another factor to consider. Esperion’s Nexletol and Nexlizet target a crowded cholesterol management market. Although there is a significant patient population, several companies market its drugs for lowering cardiovascular risks by reducing LDL-C levels. Key players in this indication include Sanofi SNY and Amgen’s AMGN PCSK9 inhibitors, Praluent and Repatha, respectively. Both Sanofi and Amgen have significant resources at their disposal, compared with Esperion. Multiple companies are also marketing LDL-C lowering therapies, including Alnylam/Novartis’ NVS Leqvio. Esperion’s drugs are also set to face competition from generics of several branded drugs as well as statin-based therapies.
Amgen’s Repatha gained approval to include the cardiovascular indication (based on the FOURIER outcomes study) in its label in 2017. With the inclusion of the FOURIER data, patient access to Repatha has improved and the product has shown an increase in sales trajectory. Amgen’s Repatha surpassed $1 billion in sales in 2021 as well as in 2022 and is expected to grow into a multi-billion-dollar franchise through 2030.
Sanofi’s Praluent is approved in adults with CV diseases to reduce LDL-C and in addition with other LDL-lowering treatments in adults with a type of high cholesterol called homozygous familial hypercholesterolemia who need additional lowering of LDL-C.
Novartis and partner, Alnylam’s Leqvio (inclisiran) has been approved in Europe for the treatment of adults with hypercholesterolemia or mixed dyslipidemia. The drug was approved by the FDA to reduce LDL-C with two doses a year. It is indicated in the United States as an adjunct to diet and maximally tolerated statin therapy for treating atherosclerotic cardiovascular disease or HeFH in adult patients requiring additional lowering of LDL-C.
In November 2022, Novartis announced results from the phase II open-label extension ORION-3 trial, which showed that Leqvio provides effective LDL-C reduction over a four-year period in patients with either ASCVD or ASCVD risk equivalent and elevated LDL-C despite maximally tolerated statin therapy.
Esperion Therapeutics, Inc. Price and Consensus

Esperion Therapeutics, Inc. price-consensus-chart | Esperion Therapeutics, Inc. Quote
Zacks Rank
Esperion currently has a Zacks Rank #3 (Hold). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Sanofi (SNY) : Free Stock Analysis Report
Novartis AG (NVS) : Free Stock Analysis Report
Amgen Inc. (AMGN) : Free Stock Analysis Report
Esperion Therapeutics, Inc. (ESPR) : Free Stock Analysis Report

 Yahoo Finance
Yahoo Finance 