Incyte (INCY) Vitiligo Drug Povorcitinib Positive in Phase II
Incyte INCY announced new data from a phase IIb study evaluating the safety and efficacy of povorcitinib (INCB54707), which is being evaluated for the treatment of extensive nonsegmental vitiligo in adults.
Vitiligo is a chronic autoimmune disease characterized by the depigmentation of the skin that results from the loss of pigment-producing cells known as melanocytes.
The phase IIb randomized, double-blind, placebo-controlled, dose-ranging study is evaluating the efficacy and safety of povorcitinib (formerly INCB54707) in adult patients with extensive nonsegmental vitiligo.
The study enrolled 171 patients (age 18 to 75 years) diagnosed with nonsegmental vitiligo affecting ≥8% of their body surface area and randomized them 1:1:1:1 to receive once-daily (QD) povorcitinib 15 mg (n=43), 45 mg (n=41), 75 mg (n=42), or placebo (n=42) for 24 weeks during the placebo-controlled period. Among these, 168 patients were treated as part of the 24-week placebo-controlled period.
The primary endpoint is the percentage change from baseline in the total body Vitiligo Area Scoring Index (T-VASI) at week 24. The key secondary endpoint is the percentage of patients achieving T-VASI50 (≥50% reduction from baseline in the T-VASI) at week 24.
Results from the study showed that treatment with oral povorcitinib was associated with substantial total body repigmentation in patients with extensive nonsegmental vitiligo, as measured by total T-VASI scores. The investigational therapy was well tolerated and the study met its primary endpoint.
Povorcitinib (INCB54707) is an oral small-molecule JAK1 inhibitor. The candidate is currently in phase II studies for vitiligo, hidradenitis suppurativa (HS) and prurigo nodularis. Phase III studies in HS are also ongoing.
Shares of Incyte have lost 3.8% in the past year compared with the industry’s decline of 15.5%.
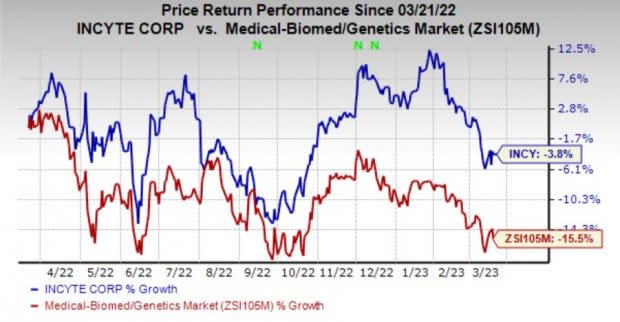
Image Source: Zacks Investment Research
Incyte also announced new 104-week results from the phase III TRuE-V clinical trial program evaluating Opzelura (ruxolitinib) cream 1.5% in patients 12 years of age and older with nonsegmental vitiligo. Patients achieving a high level of facial repigmentation at week 52 maintained a durable response a year following the withdrawal of treatment. The continued treatment with Opzelura for up to two years resulted in sustained facial repigmentation and further improvements in facial and total body repigmentation.
Opzelura, a novel cream formulation of Incyte’s selective JAK1/JAK2 inhibitor ruxolitinib, approved by the FDA for the topical treatment of nonsegmental vitiligo in patients 12 years of age and older.
The successful development of other drugs will add an incremental stream of revenues to the top line and reduce the company’s dependence on Jakafi.
Incyte has a collaboration agreement with Swiss pharma giant Novartis NVS for Jakafi.
Jakafi is marketed by Incyte in the United States and by Novartis as Jakavi outside the country.
Zacks Rank and Stocks to Consider
Incyte currently carries a Zacks Rank #3 (Hold). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
A couple of better-ranked stocks in the healthcare sector are Novo Nordisk NVO and Ligand Therapeutics LGND, both carrying a Zacks Rank #1 at present.
In the past 30 days, estimates for Novo Nordisk’s 2023 earnings per share have risen from $4.20 to $4.43 and estimates for 2024 have gone up by 29 cents to $5.19.
Ligand’s earnings per share estimates for 2023 increased to $4.15 from $3.30 in the past 30 days. LGND beat earnings estimates in one of the last four reported quarters.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Novartis AG (NVS) : Free Stock Analysis Report
Novo Nordisk A/S (NVO) : Free Stock Analysis Report
Incyte Corporation (INCY) : Free Stock Analysis Report
Ligand Pharmaceuticals Incorporated (LGND) : Free Stock Analysis Report

 Yahoo Finance
Yahoo Finance 