Intercept's (ICPT) NDA for NASH Treatment Accepted By FDA
Intercept Pharmaceuticals, Inc. ICPT announced that the FDA has accepted the company’s new drug application (NDA) for obeticholic acid (OCA) that seeks accelerated approval for the treatment of patients with pre-cirrhotic liver fibrosis due to nonalcoholic steatohepatitis (NASH).
The FDA indicated that it considers this a complete Class 2 resubmission and has assigned a target action date of Jun 22, 2023, for the NDA.
The NDA, submitted last month, is supported by two positive interim 18-month analyses from the phase III REGENERATE study in patients with pre-cirrhotic liver fibrosis due to NASH. Data showed OCA 25 mg consistently demonstrated double the response rate of placebo in reduction in liver fibrosis stage without worsening of any of the three histologic components of NASH, an endpoint consistent with the FDA’s draft guidance. Additionally, a detailed assessment of 2,477 patients in REGENERATE, including nearly 1,000 patients on a study drug for at least four years, provides a well-characterized safety profile that is monitorable and manageable and supports the chronic administration of OCA.
Intercept Pharmaceuticals, Inc. Price and Consensus

Intercept Pharmaceuticals, Inc. price-consensus-chart | Intercept Pharmaceuticals, Inc. Quote
In June 2020, Intercept received a complete response letter (“CRL”) from the FDA stating that its NDA for OCA for the treatment of liver fibrosis due to NASH could not be approved in its present form. The CRL indicated that the FDA has determined that the predicted benefit of OCA, based on a surrogate histopathologic endpoint, remains uncertain and does not sufficiently outweigh the potential risks to support accelerated approval for the treatment of patients with liver fibrosis due to NASH. This analysis was based on the data reviewed by the agency. The FDA then recommended that Intercept submit additional post-interim analysis efficacy and safety data from the ongoing REGENERATE study in support of potential accelerated approval and that the long-term outcomes phase of the trial should continue.
OCA is approved under the brand name Ocaliva for treating primary biliary cholangitis (PBC) in combination with ursodeoxycholic acid (UDCA) in adults with an inadequate response to UDCA alone or as a monotherapy for adults intolerant to UDCA.
Intercept’s shares have gained 6.4% in the last six months compared with the industry’s growth of 1.7%.
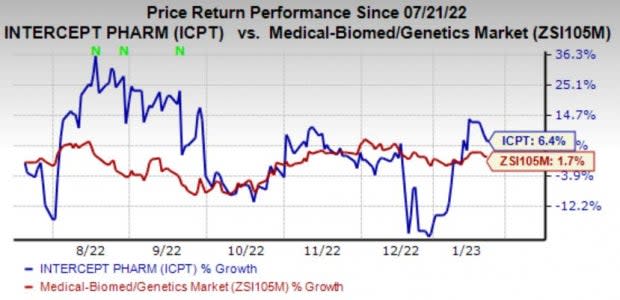
Image Source: Zacks Investment Research
The successful development of OCA for NASH will give a significant boost to Intercept.
While the NASH market promises potential with no approved therapies yet, it is challenging.
Quite a few players are trying their hand at successfully developing a treatment for the same condition.
Madrigal Pharmaceuticals, Inc. MDGL skyrocketed significantly on Dec 19 after the company announced positive top line results from the phase III MAESTRO-NASH biopsy clinical trial of resmetirom, a liver-directed selective thyroid hormone receptor agonist. The study achieved both liver histological improvement endpoints as proposed by the FDA, which is likely to predict clinical benefit for supporting accelerated approval of resmetirom to treat NASH with liver fibrosis. Madrigal intends to file an NDA seeking accelerated approval of resmetirom for treating non-cirrhotic NASH with liver fibrosis.
Earlier this month, Chemomab Therapeutics, Ltd. CMMB announced that its mid-stage study evaluating CM-101 in non-alcoholic steatohepatitis (NASH) patients was successful. The trial met its primary endpoint of safety and tolerability, and CM-101 achieved reductions in secondary endpoints that include a range of liver fibrosis biomarkers and physiologic assessments measured at baseline and at week 20. Shares of Chemomab gained on the same.
Intercept currently carries a Zacks Rank #3 (Hold). You can see the complete list of today’s Zacks #1 Rack (Strong Buy) stocks here.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Intercept Pharmaceuticals, Inc. (ICPT) : Free Stock Analysis Report
Madrigal Pharmaceuticals, Inc. (MDGL) : Free Stock Analysis Report
Chemomab Therapeutics Ltd. Sponsored ADR (CMMB) : Free Stock Analysis Report

 Yahoo Finance
Yahoo Finance 