Ironwood (IRWD) Q1 Earnings Beat Estimates, Linzess Volume Up
Ironwood Pharmaceuticals, Inc. IRWD reported first-quarter 2023 adjusted earnings of 25 cents per share, which beat both the Zacks Consensus Estimate and our model estimates of 24 cents and 23 cents, respectively. In the year-ago quarter, the company reported earnings of 21 cents per share.
Total revenues of $104 million also beat the Zacks Consensus Estimate and our model estimates of $100 million and $99.5 million, respectively. The top line increased 7% year over year.
The company’s shares were up almost 4.5% on Thursday, after trading hours, following better-than-expected earnings results. The stock nosedived 14.6% in the year-to-date period compared with the industry’s 0.3% decline.
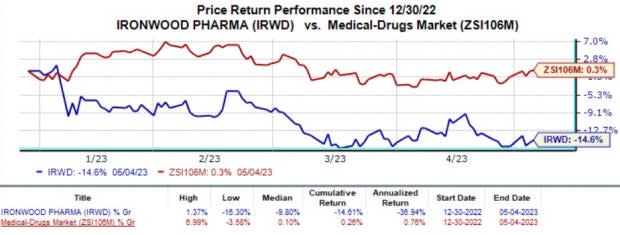
Image Source: Zacks Investment Research
Quarter in Detail
As reported by partner AbbVie ABBV, Ironwood’s sole marketed product — Linzess (linaclotide) — generated net sales of almost $250.2 million in the United States, up 8% year over year. Ironwood and AbbVie equally shar Linzess’ brand collaboration profits and losses.
IRWD’s share of net profits from the sales of Linzess in the United States (included in collaborative revenues) was $101.6 million, up 8% year over year.
The performance can be attributed to acceleration in new prescription volume. The new prescription demand increased 10% from that recorded in the year-ago period.
The company recorded $2.5 million in royalties and other revenues, down 22% from the prior-year quarter’s figure.
Ironwood has agreements with two partners — Astellas Pharma and AstraZeneca AZN — related to the development and commercialization of Linzess in Japan and China, respectively. Both Astellas and AstraZeneca have exclusive rights to develop and market the drug in their respective territories. The partners are liable to pay royalties to Ironwood on net Linzess revenues earned in their own regions.
Selling, general and administrative expenses were up 7.6% year over year to $31.1 million. Research and development expenses also increased 19% from $12.9 million in the corresponding period of 2022.
2023 Guidance
Ironwood has maintained its previously issued guidance for 2023. The company expects total revenues in the range of $420-$435 million. It also anticipates U.S. sales of Linzess to grow in the band of 3-5%.
The company expects adjusted EBITDA to exceed $250 million for the year.
Pipeline Updates
Ironwood is also focused on the label expansion of Linzess, which will help drive its sales following successful development and approval.
The FDA granted a priority review to a supplemental new drug application (sNDA), seeking expanded use of Linzess in children and adolescents with functional constipation. A final decision on the sNDA is expected on Jun 14, 2023. Currently, there is no FDA-approved therapy for children with functional constipation.
Other than Linzess, Ironwood is also evaluating two other pipeline candidates, IW-3300 and CNP-104, for treating visceral pain conditions and primary biliary cholangitis (PBC), respectively. The phase II proof-of-concept study evaluating IW-3300 is ongoing in interstitial cystitis/bladder pain syndrome.
IRWD, in collaboration with COUR Pharmaceuticals, is developing CNP-104. The clinical study for the same is being conducted by COUR Pharmaceuticals to evaluate its safety, tolerability, pharmacodynamic effects and efficacy in PBC patients. The study’s data is expected to be available in the second half of 2023. If successful, CNP-104 has the potential to be the first PBC disease-modifying therapy.
Ironwood Pharmaceuticals, Inc. Price and EPS Surprise
Ironwood Pharmaceuticals, Inc. price-eps-surprise | Ironwood Pharmaceuticals, Inc. Quote
Zacks Rank & Stock to Consider
Currently, Ironwood has a Zacks Rank #4 (Sell).
A better-ranked stock for investors interested in the same sector is Ocuphire Pharma OCUP, sporting a Zacks Rank #1(Strong Buy) at present. You can see the complete list of today’s Zacks #1 Rank stocks here.
Loss per share estimates for Ocuphire Pharma have narrowed from 29 cents to 24 cents for 2023 and from 86 cents to 81 cents for 2024, in the past 60 days. The company’s shares have surged 66% in the year-to-date period. Ocuphire’s earnings beat estimates in three of the last four quarters and missed the mark in one, the average surprise being 23.85%.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
AstraZeneca PLC (AZN) : Free Stock Analysis Report
Ironwood Pharmaceuticals, Inc. (IRWD) : Free Stock Analysis Report
AbbVie Inc. (ABBV) : Free Stock Analysis Report
Ocuphire Pharma, Inc. (OCUP) : Free Stock Analysis Report

 Yahoo Finance
Yahoo Finance 