Merck (MRK) COVID Pill Less Effective in Final Study, Stock Down
Shares of Merck MRK were down around 4% on Monday after announcing final data from a phase III study on it and partner Ridgeback Biotherapeutics’ oral antiviral medicine, molnupiravir.
Data from the final analysis of the MOVe-OUT study showed that the medicine reduced the risk of hospitalization or death by approximately 30% in non-hospitalized at-risk adult patients with mild or moderate COVID-19, which was less than 50% as previously reported, per interim data announced in October.
Final data from all enrolled participants from the study showed that 6.8% of patients treated with molnupiravir were hospitalized or died versus 9.7% of placebo-treated patients. There was one death reported in patients treated with molnupiravir versus nine deaths in placebo-treated patients.
Merck’s stock has declined 3.3% this year so far against an increase of 14.2% for the industry.
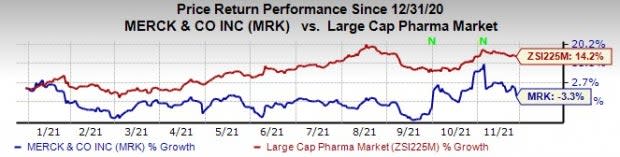
Image Source: Zacks Investment Research
Merck has already filed an application with the FDA seeking Emergency Use Authorization (EUA) for molnupiravir to treat mild-to-moderate COVID-19 in at-risk adults. The latest additional data has been shared with the FDA and will be presented at the FDA’s Antimicrobial Drugs Advisory Committee on Nov 30.
Meanwhile, in Europe, the European Medicines Agency has begun a rolling review of Merck/ Ridgeback Biotherapeutics’ regulatory application for molnupiravir. Molnupiravir was authorized in the United Kingdom, its first authorization in the world, earlier this month for treating mild-to-moderate COVID-19 patients at risk of progressing to severe illness.
Merck expects to produce 10 million courses of molnupiravir by the end of 2021 and believes the treatment’s global opportunity to be approximately $5 billion to $7 billion through 2022, including $0.5 billion to $1 billion expected to be realized in 2021.
Pfizer PFE has also made an oral antiviral candidate, Paxlovid, for COVID-19. Earlier this month, Pfizer filed an application with the FDA seeking EUA for Paxlovid for the treatment of mild-to-moderate COVID-19 in patients at increased risk of hospitalizations or death.
In clinical studies, Paxlovid reduced the risk of hospitalization or death by 89% compared to placebo in an interim analysis of phase II/III study. Pfizer has also begun rolling submissions in several countries, including the United Kingdom, Australia, New Zealand and South Korea.
At present, mild-to-moderate COVID-19 patients are being treated with monoclonal antibodies from companies like Regeneron Pharmaceuticals REGN and Eli Lilly LLY. But these need to be administered in a hospital intravenously or by subcutaneous infusion.
Lilly’s COVID-19 antibody cocktail, bamlanivimab plus etesevimab, was granted emergency approval by the FDA in February 2021 to treat mild-to-moderate COVID-19 in high-risk patients based on data from the BLAZE-1 study.
In September, the FDA expanded the EUA for the cocktail antibody medicine to include the post-exposure prevention (prophylaxis) for COVID-19 indication. Lilly’s cocktail medicine generated revenues of $217.1 million in the third quarter of 2021.
Regeneron’s antibody cocktail, REGEN-COV comprises two monoclonal antibodies, casirivimab and imdevimab and has become a significant contributor to its top line in recent quarters. Regeneron’s antibody cocktail, REGEN-COV, generated total sales of $1.2 billion in the third quarter of 2021.
If approved (emergency) by the FDA, Paxlovid and molnupiravir can be the first oral antiviral medicines prescribed as at-home treatments for mild-to-moderate COVID-19 to reduce the severity of the disease. It can help prevent hospitalization in patients with a mild-to-moderate form of the disease but at high risk of severe COVID-19.
Merck currently carries a Zacks Rank #3 (Hold). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Regeneron Pharmaceuticals, Inc. (REGN) : Free Stock Analysis Report
Pfizer Inc. (PFE) : Free Stock Analysis Report
Merck & Co., Inc. (MRK) : Free Stock Analysis Report
Eli Lilly and Company (LLY) : Free Stock Analysis Report
To read this article on Zacks.com click here.

 Yahoo Finance
Yahoo Finance 