Novavax (NVAX) Stock Nosedives 88% This Year: Here's Why
Shares of Novavax, Inc. NVAX have slumped 88.4% so far this year compared with the industry’s 19.5% decline.

Image Source: Zacks Investment Research
One of the primary reasons for this substantial decline was the delayed launch of the company’s protein-based COVID-19 vaccine, NVX-CoV2373, compared with its peers like Pfizer PFE/BioNTech BNTX and Moderna MRNA. These companies’ vaccines dominate the COVID vaccine space. Novavax’s COVID-19 vaccine is also its first marketed product.
The FDA filing, which was supposed to have been done by management in May 2021, was completed after several delays in January 2022. This delayed filing resulted in a substantial loss of market share for Novavax. Though the vaccine was granted FDA’s authorization for adults in July 2022, most individuals in the country have already been inoculated with an mRNA-based COVID vaccine by Pfizer/BioNTech and Moderna.
Though the vaccine has also been authorized for use in adolescents and as a third/booster dose in adults, it has been severely lagging behind competition and dominance imposed by Pfizer/BioNTech and Moderna’s mRNA vaccines. This was also one of the reasons management slashed the company’s total revenue guidance for 2022 twice, down to $2 billion from the original range of $4-$5 billion.
Compared to Novavax’s COVID vaccine, mRNA-based vaccines have high efficacy rates. The COVID-19 vaccines developed by Modernaand Pfizer/BioNTech are presently the only COVID-19 vaccines that have received full approval in the United States. While Moderna’s vaccine is approved for adults, the Pfizer/BioNTech vaccine is approved for use in adults and adolescents. Both these vaccines are also authorized for use in individuals aged six months and above in the United States.
Novavax is also lagging in the development of Omicron-specific vaccines. Though a late-stage study evaluating its Omicron BA.1 vaccine candidate met the primary strain-change endpoint, the candidate is yet to be launched in the market. In this regard, Moderna and Pfizer/BioNTech have already secured emergency use authorization (EUA) from the FDA for the use of their respective Omicron BA.4/BA.5-adapted bivalent vaccines as a single booster dose in individuals as young as five years of age.
Earlier this month, Novavax announced that it sent a written notice to GAVI Alliance on Nov 18 to terminate the advance purchase agreement (APA) entered between the two organizations last year for the supply of 350 million doses of its COVID vaccine. Per Novavax, GAVI has breached the APA since it failed to procure the required vaccine doses from the company for the COVAX facility. Management has also stated that GAVI has no right to recover any advance payment made by it to the company under the APA. Per Novavax, GAVI has paid a total of $700 million as a non-refundable advance payment.
Reports suggest that GAVI rejected this claim and stated that Novavax was unable to make delivery of a single dose of the protein-based COVID vaccine to COVAX. The company also stated that it would recover the advanced payments made to Novavax made under the APA. A legal battle is most likely to ensue between Novavax and GAVI as both contradict each other’s claims, which may hurt the stock further if there is any negative development in this regard.
Novavax, Inc. Price
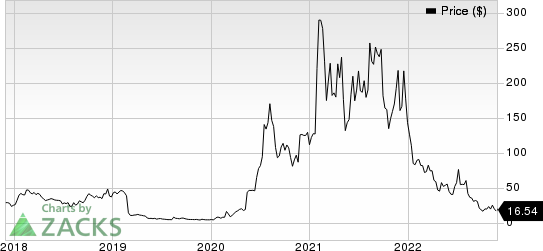
Novavax, Inc. price | Novavax, Inc. Quote
Zacks Rank
Novavax currently carries a Zacks Rank #4 (Sell). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Pfizer Inc. (PFE) : Free Stock Analysis Report
Moderna, Inc. (MRNA) : Free Stock Analysis Report
Novavax, Inc. (NVAX) : Free Stock Analysis Report
BioNTech SE Sponsored ADR (BNTX) : Free Stock Analysis Report

 Yahoo Finance
Yahoo Finance 