PerkinElmer's (PKI) New Viral Vector Assays to Boost Workflows
PerkinElmer, Inc. PKI recently launched ready-to-use Adeno-associated Virus Vectors (AAV) Detection Kits to aid researchers working on gene therapies for various serious diseases. The validated and fully-automatable assays are built on PerkinElmer's proprietary AlphaLISA technology.
The latest launch will likely expand PerkinElmer's cell and gene therapy portfolio, which includes gene editing and modulation, cell counting, antibody and flow cytometry innovations. This will enable the company to significantly strengthen its Life Sciences business unit in the broader Discovery & Analytical Solutions segment.
Significance of the Launch
The high-throughput viral assays have been designed to aid researchers to quickly and easily characterize viral vector particles being produced to enable decision-making for safe and efficient gene transfer. As the assays are built on PerkinElmer's proprietary AlphaLISA technology, it does not require separation. These are the only optimized, no-wash AAV detection assays available on the market. The new offering provides researchers expanded options to measure viral titers beyond ELISA and other wash-based systems, which can be time-consuming and limited in assay range.
Each of the seven kits, designed to streamline gene therapy research and development workflows with a user-friendly and higher throughput method, detects specific serotypes to target different cell types in the body for gene therapy application.
Per management, the new AAV detection kits have been engineered to provide solutions to gene therapy researchers to aid them navigate the unique workflows they work with to reduce and simplify the path from lab to the clinic. This is expected to be achieved by removing long, tedious protocols while expanding the detection range to enable potential cures for people living with cancer and Alzheimer’s, among others.
Industry Prospects
Per a report by Research and Markets published on GlobeNewswire, the global cell and gene therapy market is expected to reach from $6.58 billion in 2021 to $21.33 billion in 2026 at a CAGR of 25.6%. Factors like increasing incidences of rare and chronic diseases, advancements in cell and gene therapy and a rising number of clinical trials are likely to drive the market.
Given the market potential, the latest launch is expected to strengthen PerkinElmer’s global business.
Recent Developments
This month, PerkinElmer received the FDA’s marketing authorization for the EONIS SCID-SMA assay kit for in vitro diagnostic use by certified laboratories for the simultaneous detection of spinal muscular atrophy (SMA) and severe combined immunodeficiency (SCID) in newborns. Other components of the platform include the EONIS DNA Extraction kit and EONIS Analysis Software.
This month, PerkinElmer announced its third-quarter 2022 results, where it reported better-than-expected pro-forma adjusted earnings per share. Per management, the company is well-poised to execute its short-term and long-term goals on the back of perseverance and team effort.
PerkinElmer, in September, launched the Cellaca PLX Image Cytometry System, a first-of-its-kind benchtop platform that enables researchers to assess multiple Critical Quality Attributes of cell samples in a single automated workflow, including cell identity, quality and quantity.
Price Performance
Shares of the company have lost 22.8% in the past year compared with the industry’s 13.9% decline and the S&P 500's 17.5% fall.
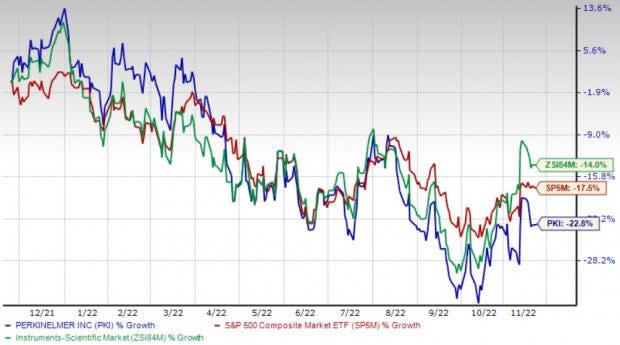
Image Source: Zacks Investment Research
Zacks Rank & Key Picks
Currently, PerkinElmer carries a Zacks Rank #3 (Hold).
Some better-ranked stocks in the broader medical space are AMN Healthcare Services, Inc. AMN, ShockWave Medical, Inc. SWAV and McKesson Corporation MCK.
AMN Healthcare, carrying a Zacks Rank #2 (Buy) at present, has an estimated long-term growth rate of 3.3%. AMN’s earnings surpassed the Zacks Consensus Estimate in all the trailing four quarters, the average beat being 10.9%.
You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
AMN Healthcare has gained 8.6% against the industry’s 33.7% decline in the past year.
ShockWave Medical, carrying a Zacks Rank #2 at present, has an estimated growth rate of 23.6% for 2023. SWAV’s earnings surpassed estimates in all the trailing four quarters, the average beat being 146.1%.
ShockWave Medical has gained 30.1% against the industry’s 27.4% decline over the past year.
McKesson, carrying a Zacks Rank #2 at present, has an estimated long-term growth rate of 10.1%. MCK’s earnings surpassed estimates in two of the trailing four quarters and missed the same in the other two, the average beat being 4.8%.
McKesson has gained 67.1% against the industry’s 12.2% decline over the past year.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
McKesson Corporation (MCK) : Free Stock Analysis Report
PerkinElmer, Inc. (PKI) : Free Stock Analysis Report
AMN Healthcare Services Inc (AMN) : Free Stock Analysis Report
ShockWave Medical, Inc. (SWAV) : Free Stock Analysis Report

 Yahoo Finance
Yahoo Finance 