Pharma Stock Roundup: ABBV's Deal With RGNX, LLY COVID Drug's Government Order Win
AbbVie ABBV is set to co-develop Regenexbio's RGNX investigational gene therapy for chronic retinal diseases like wet age-related macular degeneration. Eli Lilly & Company LLY announced a new agreement with the U.S. government to supply additional doses of its COVID-19 antibody drug, etesevimab amid greater demand due to rising infection rates in the country.
Recap of the Week’s Most Important Stories
AbbVie Buys Rights to Eye Disease Gene Therapy from Regenexbio: AbbVie announced a deal to co-develop and co-commercialize Regenexbio's investigational gene therapy for chronic retinal diseases like wet age-related macular degeneration. AbbVie will make a $370 million upfront payment to Regenxbio for rights to RGX-314. Additionally, Regenxbio will also be entitled to milestone payments of up to $1.38 billion. While Regenxbio will be responsible for the completion of the ongoing studies of RGX-314, AbbVie will share costs of future studies, which include a second pivotal study for wet AMD utilizing subretinal delivery.
AbbVie submitted regulatory applications in the United States and Europe seeking approval for Rinvoq (upadacitinib) for the treatment of adults with moderately to severely active ulcerative colitis. The label expansion applications were based on data from the two phase III induction studies and one maintenance study. While Rinvoq is approved in the United States for rheumatoid arthritis (RA), in Europe, the drug is approved for certain patients with RA, active psoriatic arthritis, atopic dermatitis, and active ankylosing spondylitis.
U.S. Government Orders Additional COVID-19 Drugs from Lilly: Lilly announced a deal to supply 388,000 doses of its COVID-19 antibody medicine, etesevimab to the U.S. government. These doses will complement the doses of bamlanivimab that the U.S. government has already purchased. Please note that bamlanivimab plus etesevimab is approved for emergency use as a cocktail medicine to treat mild-to-moderate COVID-19 in high-risk patients. None of the antibody drugs are approved for monotherapy use. Bamlanivimab’s Emergency Use Authorization (EUA) as a monotherapy treatment for COVID was revoked by the FDA in April at Lilly’s request.
Separately, the FDA expanded the cocktail medicine’s EUA to include the post-exposure prevention (prophylaxis) for COVID-19 indication. This means the cocktail medicine can now be used (on an emergency basis) to treat certain people who have been exposed to someone infected with SARS-CoV-2 or who are at high risk of exposure in an institutional setting, including a nursing home or prison. The expanded authorization is based on data from BLAZE-2, which showed that bamlanivimab significantly reduced the risk of contracting symptomatic COVID-19 in residents and staff at long-term care facilities/nursing homes.
Theravance Hypotension Study Fails, to Cut Workforce: Theravance Biopharma’s TBPH phase III study evaluating its investigational pipeline candidate, ampreloxetine, for the treatment of symptomatic neurogenic orthostatic hypotension (nOH) did not meet the primary endpoint as the candidate failed to show an improvement in OHSA #1 in patients. The company announced a major cost-reduction program and undertook strategic actions to prioritize its marketed and investigational respiratory medicines. Theravance is looking to reduce its workforce by 75%, an estimated 270 job cuts. With this strategic implementation, Theravance is looking to make annualized cost savings of approximately $165 million in 2022.
Aerie’s Dry Eye Candidate Misses Main Goal in Study:Aerie Pharmaceuticals AERI announced data from the phase IIb study (“COMET-1”), which evaluated its novel dry eye product candidate, AR-15512. While the study achieved statistical significance over multiple pre-specified symptom and sign endpoints, it did not achieve all pre-determined primary endpoints with statistical significance.
The NYSE ARCA Pharmaceutical Index declined 1.5% in the last five trading sessions.
Large Cap Pharmaceuticals Industry 5YR % Return
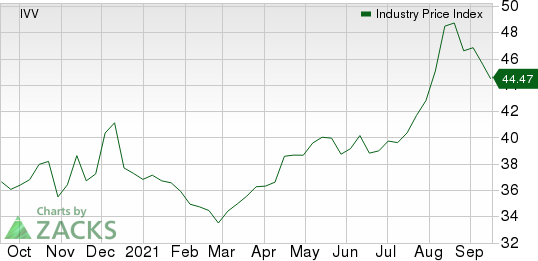
Large Cap Pharmaceuticals Industry 5YR % Return
Here’s how the eight major stocks performed in the last five trading sessions.
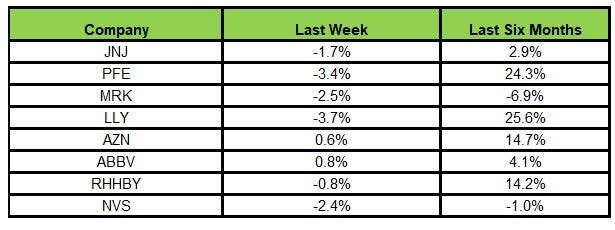
Image Source: Zacks Investment Research
In the last five trading sessions, AbbVie rose the most (0.8%) while Lilly declined the most (3.7%)
In the past six months, Lilly recorded the maximum gain (25.6%) while Merck MRK declined the most (6.9%)
(See the last pharma stock roundup here: SNY to Buy Kadmon, New Approvals for AZN & MRK’s Drugs)
What's Next in the Pharma World?
Watch out for regular pipeline and regulatory updates next week.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Merck & Co., Inc. (MRK) : Free Stock Analysis Report
Eli Lilly and Company (LLY) : Free Stock Analysis Report
AbbVie Inc. (ABBV) : Free Stock Analysis Report
Theravance Biopharma, Inc. (TBPH) : Free Stock Analysis Report
Aerie Pharmaceuticals, Inc. (AERI) : Free Stock Analysis Report
REGENXBIO Inc. (RGNX) : Free Stock Analysis Report
To read this article on Zacks.com click here.
Zacks Investment Research

 Yahoo Finance
Yahoo Finance 