Pharma Stock Roundup: EU Nod to AZN & RHHBY Drugs, FDA Updates for LLY & MRK
This week, the European Commission (EC) granted approval to Roche’s RHHBY eye injection, Vabysmo and AstraZeneca’s AZN asthma drug, Tezspire. The FDA approved expanded use of Eli Lilly’s LLY cancer drug Retevmo for multiple tumor types and allowed Merck MRK to resume a phase III program on HIV candidate islatravir with a lower dose. GSK GSK signed an exclusive license agreement with Spero Therapeutics for its investigational antibiotic for complicated urinary tract infections (cUTI).
Recap of the Week’s Most Important Stories
Roche’s Eye Injection, Vabysmo Gets Approval in Europe: The EC granted approval to Roche’s injectable eye medicine, Vabysmo (faricimab) for treating two leading causes of vision loss worldwide — neovascular or wet age-related macular degeneration (nAMD) and visual impairment due to diabetic macular edema (DME). The approval was based on data from four phase III studies in nAMD and DME. Vabysmo was approved by the FDA for nAMD and DME in January.
EU Approves AstraZeneca’s Tezspire: The EC also approved AstraZeneca’s monoclonal antibody, Tezspire (tezepelumab), for the treatment of severe asthma. Tezspire was approved in the United States for a similar indication in December 2021. AstraZeneca markets Tezspire in partnership with Amgen. The approval of Tezspire was based on data from the PATHFINDER clinical program and included the pivotal phase III NAVIGATOR study. Data from these studies showed that treatment with Tezspire led to superior results across every primary and key secondary endpoint in patients with severe asthma, compared to placebo, when added to standard therapy.
The EC also granted marketing approval to AstraZeneca’s antibody cocktail medicine, Evusheld in the European Union for the treatment of COVID-19. The recommendation was based on TACKLE phase III treatment data, which showed a reduced risk of severe COVID-19 or death on treatment with one intramuscular dose of Evusheld.
Evusheld was until now authorized for the prevention (pre-exposure prophylaxis) of COVID-19 in certain high-risk populations in Europe as well as the United States and some other countries. The medicine was not authorized for treating COVID-19. Evusheld is now the only long-acting antibody combination available for both prevention and treatment of COVID-19 in Europe.
FDA Approves Lilly’s Retevmo for Multiple RET-Driven Tumor Types: The FDA approved Lilly’s cancer drug Retevmo for expanded use in locally advanced or metastatic solid tumors with a RET gene fusion. Until now, Retevmo was approved for patients with advanced RET-driven lung and thyroid cancers. The latest approval of the tumor-agnostic indication expands Retevmo’s patient base to include several tumor types. The approval is based on positive data from the pivotal phase I/II LIBRETTO-001 study, which evaluated selpercatinib across a variety of tumor types in patients with RET-driven cancers. In the study, Retevmo demonstrated an overall response rate of 44% across multiple tumor types.
Simultaneously, the FDA converted the accelerated approval granted to Retevmo in non-small cell lung cancer indication in May 2020 to a full approval.
FDA Allows Merck to Resume Islatravir HIV Studies with Lower Dose: Merck announced that it will evaluate a lower dose of its HIV candidate islatravir in a phase III program several months after the FDA put studies on the candidate on hold last year. While six studies on islatravir were placed on full clinical hold, seven were put on partial clinical hold. Now the new phase III studies will evaluate a once-daily oral combination of doravirine 100 mg and a lower dose of islatravir (DOR/ISL). Merck will also resume a phase II study evaluating the combination of a once-weekly treatment regimen of islatravir and Gilead’s lenacapavir. Even this study will use a lower dose of islatravir. However, Merck has decided to discontinue the development of once-monthly oral islatravirfor theprevention of HIV infection, also called pre-exposure prophylaxis or PrEP. The FDA has agreed to Merck’s plan.
GSK Buys Exclusive Rights to Spero’s Novel Oral Antibiotic: GSK is in-licensing exclusive rights to develop and commercialize Spero Therapeutics’ late-stage oral antibiotic candidate, tebipenem HBr for the potential treatment of cUTI. If approved by the FDA, tebipenem HBr will become the first oral treatment for cUTI, providing patients a novel alternative to in-hospital intravenous therapy
For the acquisition, Glaxo will make an upfront payment of $66 million to Spero, while the latter will also be entitled to future milestone payments and tiered royalties. In addition, Glaxo will invest $9 million in shares of Spero. Per the exclusive license deal, Glaxo will develop and commercialize tebipenem HBr in all countries except for Japan and certain other Asian countries
The NYSE ARCA Pharmaceutical Index declined 1.8% in the last five trading sessions.
Large Cap Pharmaceuticals Industry 5YR % Return

Large Cap Pharmaceuticals Industry 5YR % Return
Here’s how the eight major stocks performed in the last five trading sessions.
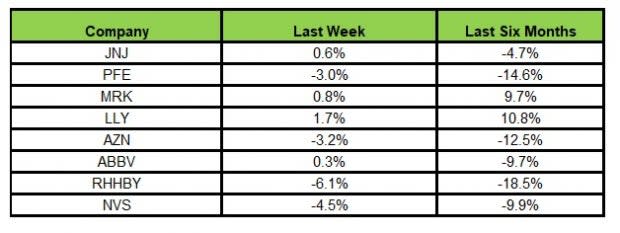
Image Source: Zacks Investment Research
In the last five trading sessions, Lilly rose the most (1.7%) while Roche declined the most (6.1%).
In the past six months, Lilly has gained the highest (10.8%) while Roche declined the most (18.5%).
(See the last pharma stock roundup here: J&J New Buyback Plan, CHMP Nod to PFE’s BA.4, BA.5 Omicron Jab)
What's Next in the Pharma World?
Watch out for regular pipeline and regulatory updates next week.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
AstraZeneca PLC (AZN) : Free Stock Analysis Report
GSK PLC Sponsored ADR (GSK) : Free Stock Analysis Report
Roche Holding AG (RHHBY) : Free Stock Analysis Report
Merck & Co., Inc. (MRK) : Free Stock Analysis Report
Eli Lilly and Company (LLY) : Free Stock Analysis Report
To read this article on Zacks.com click here.
Zacks Investment Research

 Yahoo Finance
Yahoo Finance 