Pharma Stock Roundup: FDA Panel Vote for MRK COVID Pill, JNJ Omicron Jab Plan
This week, an FDA committee voted in favor of authorizing Merck’s MRK antiviral pill, molnupiravir to treat COVID-19. J&J JNJ said it is evaluating the effectiveness of its COVID-19 vaccine against the Omicron variant. AbbVie ABBV filed an application in Europe seeking approval for Skyrizi for Crohn’s disease (“CD”). Glaxo GSK and partner Vir Biotech’s VIR monoclonal antibody drug, sotrovimab proves to be effective against the Omicron variant in preclinical studies.
Recap of the Week’s Most Important Stories
FDA Panel Recommends Merck’s COVID Pill: The FDA’s Antimicrobial Drugs Advisory Committee (AMDAC) voted 13-0 to recommend the authorization of Merck and partner Ridgeback Biotherapeutics’ oral antiviral pill, molnupiravir to treat mild-to-moderate COVID-19 in at-risk adults. The committee gave the positive vote as it felt the medicine’s potential benefits outweighed its risks. Though the FDA is not bound by the committee’s recommendation, it usually follows the same.
Merck announced final data from a phase III study on molnupiravir. Data from the final analysis of the MOVe-OUT study showed that the medicine reduced the risk of hospitalization or death by approximately 30% in non-hospitalized at-risk adult patients with mild or moderate COVID-19, which was less than 50% as previously reported, per interim data announced in October.
The FDA accepted and granted priority review to Merck and AstraZeneca’s supplemental new drug application (sNDA) seeking the expanded use of PARP inhibitor Lynparza for BRCA-mutated HER2-negative high-risk early breast cancer. With the FDA granting priority review to the sNDA, a decision is expected in the first quarter of 2022. The sNDA for the expanded indication was based on data from the OlympiA phase III study.
The European Commission granted approval to Merck’s Keytruda in combination with Eisai’s Lenvima for two different indications. The first is for the first-line treatment of adult patients with advanced RCC based on data from the CLEAR/KEYNOTE-581 study. The other is for the treatment of certain adult patients with advanced or recurrent endometrial carcinoma (EC) based on data from the KEYNOTE-775/Study 309 study. Keytruda plus Lenvima was approved for both the indications in the United States in mid-2021.
The FDA accepted and granted priority review to Merck’s supplemental biologics license application (sBLA) seeking approval of Vaxneuvance, its newly approved pneumococcal 15-valent conjugate vaccine for use in children 6 weeks through 17 years of age. The FDA is expected to give its decision on the sBLA on Apr 1, 2022.
J&J Tests Efficacy of Its COVID Vaccine Against Omicron: J&J is testing the effectiveness of its single-dose COVID-19 vaccine against the new, rapidly spreading and highly mutated Omicron variant. The company is testing the blood serum from participants of completed and ongoing booster studies for neutralizing activity against the Omicron variant. J&J is also pursuing a new vaccine against Omicron, which will be pushed to clinical development, rapidly, if needed.
J&J filed an application with the European Medicines Agency (EMA) seeking expanded use of Imbruvica as a fixed-duration combination with venetoclax (I+V) for the first-line treatment of chronic lymphocytic leukemia (CLL). The application was based on data from the pivotal phase III GLOW study.
Glaxo’s Pre-Clinical Data Shows Sotrovimab Maintains Activity Against Omicron: Glaxo and partner Vir Biotech’s pre-clinical data showed that their monoclonal antibody candidate, sotrovimab, retains activity against key mutations of the Omicron variant. The data was generated through pseudo-virus testing of specific individual mutations found in Omicron, which will be further confirmed by in-vitro pseudo-virus testing being conducted by the companies.
Glaxo and Vir Biotech’s sotrovimab is presently authorized by the brand name of Xevudy in several countries including the United States to treat high-risk COVID-19. However, it is not yet authorized in Europe. Xevudy (sotrovimab) was granted conditional marketing authorisation in Great Britain for treating symptomatic adults and adolescents with acute COVID-19 infection who are at increased risk of progressing to severe COVID infection.
AbbVie Seeks Approval for Skyrizi in Europe for Crohn’s Disease: AbbVie filed an application with the EMA seeking expanded use of risankizumab both as a 600mg intravenous (“IV”) induction and 360mg subcutaneous (“SC”) maintenance therapy, as a potential treatment for moderate to severe CD. The application was based on data from three pivotal studies, ADVANCE, MOTIVATE and FORTIFY. Risankizumab is marketed by the brand name of Skyrizi for moderate-to-severe plaque psoriasis in some countries including the United States and the European Union. It is also approved to treat active psoriatic arthritis in Europe. An application seeking approval of Skyrizi for CD is also under review in the United States.
The NYSE ARCA Pharmaceutical Index declined 1.95% in the last five trading sessions.
Large Cap Pharmaceuticals Industry 5YR % Return
Large Cap Pharmaceuticals Industry 5YR % Return
Here’s how the eight major stocks performed in the last five trading sessions.
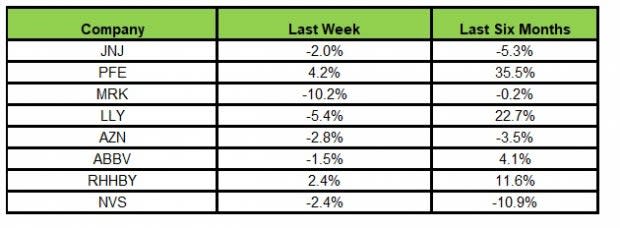
Image Source: Zacks Investment Research
In the last five trading sessions, Pfizer rose the most (4.2%) while Merck declined the most (10.2%)
In the past six months, Pfizer recorded the maximum gain (35.5%) while Novartis declined the most (10.9%)
(See the last pharma stock roundup here: FDA Nod to COVID Jab for All Adults, CHMP Nod to Shot for Kids)
What's Next in the Pharma World?
Watch out for regular pipeline and regulatory updates next week.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
GlaxoSmithKline plc (GSK) : Free Stock Analysis Report
Johnson & Johnson (JNJ) : Free Stock Analysis Report
Merck & Co., Inc. (MRK) : Free Stock Analysis Report
AbbVie Inc. (ABBV) : Free Stock Analysis Report
Vir Biotechnology, Inc. (VIR) : Free Stock Analysis Report
To read this article on Zacks.com click here.
Zacks Investment Research

 Yahoo Finance
Yahoo Finance 