QIAGEN's (QGEN) Therascreen KRAS Kit Receives FDA Approval
QIAGEN N.V. QGEN recently announced the receipt of the FDA approval for its therascreen KRAS RGQ PCR kit (therascreen KRAS kit). The kit is approved as a companion diagnostic to support detecting non-small cell lung cancer (“NSCLC”) patients eligible for treatment with KRAZATI (adagrasib).
Notably, this is the fourth approval of QIAGEN’s therascreen KRAS kit adding to the existing three therapies already indicated in the label for use in NSCLC and colorectal cancer. With the new approval, QIAGEN has 11 PCR-based companion diagnostic indications that are FDA approved — the widest portfolio of IVD approved PCR-based companion diagnostics on the market.
In May 2021, QIAGEN and Mirati Therapeutics (MRTX) announced their cooperation to accelerate the development of a tissue-based KRAS companion diagnostic, which identifies NSCLC patients with KRAS G12C.
The recent development will likely bolster QIAGEN's oncology and precision diagnostics business.
More on the News
QIAGEN is working under master collaboration contracts with over 25 companies to develop and commercialize companion diagnostic tests for their drug candidates. The company has a huge pipeline of probable future products to develop Precision Medicine for patients’ benefits.
The tissue-based KRAS companion diagnostic assay developed by QIAGEN is specifically to spot patients with NSCLC that have a KRAS G12C mutation. The drug is directed to treat adult patients with KRAS G12C-mutated locally advanced or metastatic NSCLC, as determined by an FDA-approved test, who have received at least one prior systemic therapy.
Significance of the Approval
Per management, therascreen KRAS is a quick and cost-effective test that ensures that physicians get patient reports in the most efficient and streamlined way to make treatment decisions. The approval will strengthen QIAGEN’s leadership in RAS companion diagnostics.
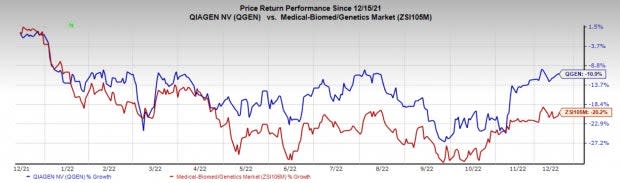
Image Source: Zacks Investment Research
The therascreen-based companion diagnostic detects KRAS G12C — a genetic mutation that is one of the most common KRAS variations related to cancer. This mutation is projected to be present in about 13% of NSCLC cases, making it the most prevalent driver mutation.
With the new approval, QIAGEN is striving to fill the clinical gap for patient access and offer technology platform options to identify varying clinical testing requirements for KRAS.
Industry Prospects
Per a report by Research and Markets, the global market for Non-Small Cell Lung Cancer Therapeutics was $22.3 billion in 2020 and is projected to reach $40.3 billion by 2027, at a CAGR of 8.9%.
Given the market potential, QIAGEN’s latest approval to aid in identifying non-small cell lung cancer is well-timed.
Recent Developments
In November 2022, QIAGEN launched monkeypox (MPXV) test for its NeuMoDx automated polymerase chain reaction (PCR) platform — NeuMoDx MPXV Test Strip — to strengthen surveillance and research into the current outbreak in non-endemic regions.
In the same month, QIAGEN announced the certification of its in-vitro diagnostic (IVD) kit and its fully automated NeuMoDx platforms under the European Union’s new In-Vitro Diagnostic Medical Devices Regulation (IVDR).
Price Performance
Shares of the company have lost 10.9% in a year compared with the industry’s fall of 20.2%.
Zacks Rank and Key Picks
Currently, QIAGEN carries a Zacks Rank #3 (Hold).
Few other better-ranked stocks in the broader medical space that investors can consider are ShockWave Medical, Inc. SWAV, Orthofix Medical Inc. OFIX and Merit Medical System MMSI.
ShockWave Medical, sporting a Zacks Rank #2 (Buy) at present, has an estimated growth rate of 33.1% for 2023. The company’s earnings surpassed estimates in all the trailing four quarters, the average beat being 180.1%.
ShockWave Medical has outperformed its industry in the past year. SWAV has gained 35% against the industry’s 32.6% fall in the past year.
Orthofix Medical currently carrying a Zacks Rank #1 (Strong Buy), reported third-quarter 2022 adjusted EPS of 13 cents, which beat the Zacks Consensus Estimate by a stupendous 550%. Revenues of $114 million outpaced the consensus mark by 2.7%.
Orthofix Medical has an estimated next-year growth rate of 58.97%. MMSI’s earnings surpassed estimates in the trailing three quarters and missed in one, the average being 129.1%. You can see the complete list of today’s Zacks #1 Rank stocks here.
Merit Medical, currently carrying a Zacks Rank of 2, reported third-quarter 2022 adjusted EPS of 64 cents, which beat the Zacks Consensus Estimate by 20.8%. Revenues of $287.2 million outpaced the consensus mark by 5.2%.
Merit Medical has an estimated long-term growth rate of 11%. MMSI’s earnings surpassed estimates in all the trailing four quarters, the average being 25.4%.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
ORTHOFIX MEDICAL INC. (OFIX) : Free Stock Analysis Report
QIAGEN N.V. (QGEN) : Free Stock Analysis Report
Merit Medical Systems, Inc. (MMSI) : Free Stock Analysis Report
ShockWave Medical, Inc. (SWAV) : Free Stock Analysis Report

 Yahoo Finance
Yahoo Finance 