Regeneron (REGN) Dupixent Meets Goal in Prurigo Nodularis Study
Regeneron Pharmaceuticals, Inc. REGN announced positive data from a pivotal phase III study that evaluated its blockbuster drug, Dupixent (dupilumab), for treating adult patients with uncontrolled prurigo nodularis, a chronic inflammatory skin disease. The study met its primary and all key secondary endpoints.
Top-line data from the phase III PRIME2 study showed that 37% of patients who were treated with Dupixent experienced a clinically meaningful reduction in itch from baseline as compared to 22% of patients in the placebo arm – the primary endpoint.
The data also showed that 58% of patients who received Dupixent experienced a clinically meaningful reduction in itch from baseline versus 20% of patients who received placebo at week 24 of treatment. Also, 45% of patients who were treated with Dupixent achieved clear or almost clear skin as compared to 16% of placebo patients at week 24.
Treatment with Dupixent led to significantly greater improvements in health-related quality of life, skin pain, as well as symptoms of anxiety and depression.
Shares of Regeneron have rallied 17.8% so far this year against the industry’s decrease of 10.8%.
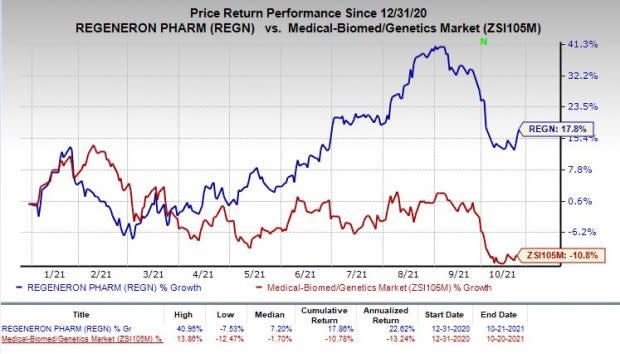
Image Source: Zacks Investment Research
We note that Dupixent is being jointly marketed by Regeneron and Sanofi SNY under a global collaboration agreement. The medicine is already approved to treat moderate-to-severe atopic dermatitis in adults as well as two other type II inflammatory diseases, namely severe chronic rhinosinusitis with nasal polyposis and severe asthma, in both the United States and Europe.
Earlier this week, the FDA approved Dupixent as an add-on maintenance therapy to treat moderate-to-severe asthma with an eosinophilic phenotype or oral corticosteroid-dependent asthma in patients aged six to 11 years.
The company has also filed a regulatory application with the European Union seeking approval for Dupixent to treat children aged six to 11 years with moderate-to-severe asthma. The application is currently under review.
Dupixent faces competition from Xolair, which is developed by Genetech, a member of the Roche Group RHHBY, and Novartis NVS to treat moderate-to-severe persistent asthma in patients aged six years and older.
Dupixent generated sales of $2.7 billion in the first six months of 2021, up 53% year over year. Label expansion approvals are driving the drug’s sales higher, with the momentum expected to continue in the future quarters as well.
Zacks Rank
Regeneron presently carries a Zacks Rank #1 (Strong Buy). You can see the complete list of today’s Zacks #1 Rank stocks here.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Regeneron Pharmaceuticals, Inc. (REGN) : Free Stock Analysis Report
Sanofi (SNY) : Free Stock Analysis Report
Novartis AG (NVS) : Free Stock Analysis Report
Roche Holding AG (RHHBY) : Free Stock Analysis Report
To read this article on Zacks.com click here.
Zacks Investment Research

 Yahoo Finance
Yahoo Finance 