Roche (RHHBY) Actemra Gets CHMP Recommendation for COVID-19
Roche RHHBY recently announced that the European Medicines Agency’s (“EMA”) Committee for Medicinal Products for Human Use (“CHMP”) has recommended extending the marketing authorization for rheumatoid arthritis drug Actemra/RoActemra (tocilizumab).
Actemra has been recommended for the treatment of COVID-19 in adults who are receiving systemic corticosteroids and require supplemental oxygen or mechanical ventilation.
The recommendation comes amid rising cases in Europe. The CHMP began an accelerated assessment of Actemra/RoActemra in August 2021.
WHO has reported that interleukin 6 receptor blockers, such as Actemra/RoActemra, are expected to still effectively manage patients with severe COVID-19 following the recent emergence of the new variant of concern, Omicron.
The recommendation was based on results from phase III studies — COVACTA, EMPACTA and REMDACTA — and the University of Oxford’s Randomised Evaluation of COVID-19 Therapy (RECOVERY) study, which was supported by Roche.
A final decision from the European Commission (“EC”) on the same is expected shortly.
In June 2021, Actemra/RoActemra received an Emergency Use Authorization (EUA) from the FDA for the intravenous treatment of COVID-19 in hospitalized adults and pediatric patients (two years of age and older) who are receiving systemic corticosteroids and require supplemental oxygen, non-invasive or invasive mechanical ventilation, or extracorporeal membrane oxygenation.
Shares of Roche have gained 13.4% so far this year compared with the industry’s growth of 12.3%.
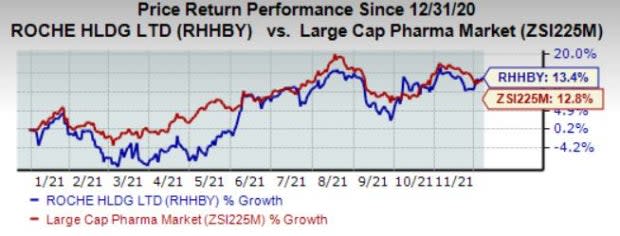
Image Source: Zacks Investment Research
Apart from launching quite a few diagnostics tests to detect the virus, Roche entered into a partnership with Regeneron REGN to jointly develop antibody cocktail Ronapreve (casirivimab and imdevimab), known as REGEN-COV in the United States.
Last month, the EC granted marketing authorization for the antibody cocktail for people aged 12 years and older for the treatment of non-hospitalized patients (outpatients) with confirmed COVID-19 who do not require oxygen supplementation and are at increased risk of progressing to severe COVID-19, and to prevent the disease.
Regeneron and Roche intend to submit a future Type II Variation to the EMA that seeks to expand the indication to include the treatment of patients hospitalized because of COVID-19.
While Regeneron invented the antibody cocktail, Roche is primarily responsible for development and distribution outside the United States.
As the pandemic continues to wreak havoc with new variants, pharma/biotech companies are striving hard to develop new ammunition for combating the same.
The FDA recently expanded EUA for Eli Lilly’s LLY bamlanivimab and etesevimab administered together to include certain high-risk pediatric patients from newborns to 12 years olds.
With the expanded EUA, Lilly’s bamlanivimab and etesevimab can now be given both for the treatment of patients with COVID-19 as well as for post-exposure prophylaxis in high-risk pediatric and infant patients (less than 12 years of age).
Roche currently carries a Zacks Rank #2 (Buy). Another top-ranked large-cap pharma stock is GlaxoSmithKline GSK, which currently carries a Zacks Rank #2. You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
GlaxoSmithKline’s earnings per share estimates for 2021 have increased from $3.02 to $3.06 in the past 30 days. The same for 2022 has increased from $3.23 to $3.26 in the past 30 days. Shares of Glaxo have risen 13.4% in the year so far.
Earnings of GlaxoSmithKline beat estimates in three of the last four quarters and missed once, with the average surprise being 15.3%.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Regeneron Pharmaceuticals, Inc. (REGN) : Free Stock Analysis Report
GlaxoSmithKline plc (GSK) : Free Stock Analysis Report
Roche Holding AG (RHHBY) : Free Stock Analysis Report
Eli Lilly and Company (LLY) : Free Stock Analysis Report
To read this article on Zacks.com click here.

 Yahoo Finance
Yahoo Finance 