Sage (SAGE), Biogen (BIIB) Report Data on Depression Drug
Sage Therapeutics, Inc. SAGE along with partner Biogen Inc. BIIB announced 12-month data from a cohort of an ongoing phase III SHORELINE study, currently evaluating their drug zuranolone in adult patients with major depressive disorder (MDD).
The SHORELINE study comprises multiple cohorts, which evaluate the safety, tolerability and the need for repeat dosing with zuranolone in adults with MDD.
This cohort of the SHORELINE study evaluated the zuranolone dose of 50 mg in 199 patients who received it once nightly for a 14-day treatment course as their initial dose and had the opportunity to be followed for 12 months.
Data from the cohort study demonstrated that zuranolone 50 mg was generally well-tolerated with no new safety findings or trends identified in the long-term safety data available to date, irrespective of the number of courses of the drug received by a patient. In fact, most patients who responded to the initial 14-day regimen received only one two-week course of treatment during the study and nearly 80% of those patients received only one or two treatment courses in total.
Sage Therapeutics’ stock has plunged 57.1% in the year so far compared with the industry’s 19.4% decline.
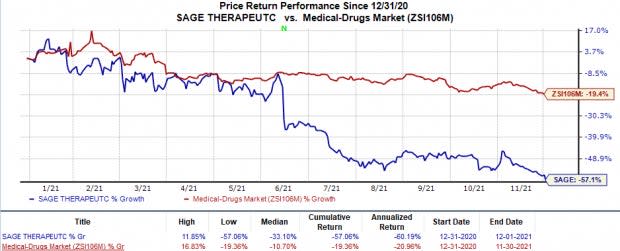
Image Source: Zacks Investment Research
Biogen’s stock has declined 6.3% in the year so far compared with the industry’s 16.1% fall.
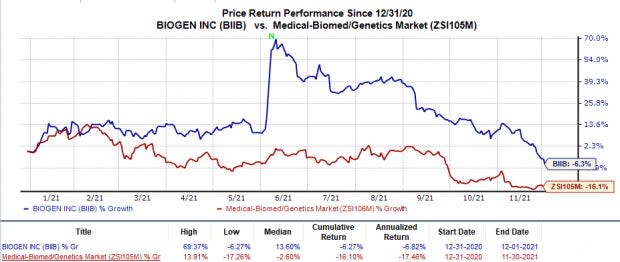
Image Source: Zacks Investment Research
Both Sage Therapeutics and Biogen already completed another cohort of the SHORELINE study, which evaluated zuranolone 30 mg as the initial dose administered once nightly for 14 days.
We note that the treatment-emergent adverse events (TEAEs) reported in patients were maximum severity of mild to moderate. The most common adverse events included somnolence, dizziness, headache, sedation, insomnia, nausea and tremor.
The SHORELINE study is part of the LANDSCAPE clinical program. Data from the cohorts of the SHORELINE study also reinforce the positive findings from the other multiple late-stage studies of the LANDSCAPE clinical program wherein zuranolone consistently demonstrated rapid and sustained improvements in depressive symptoms and a well-tolerated safety profile.
Per management, an estimated 19-million people in the United States and more than 250 million individuals worldwide experience MDD each year. Though antidepressants are widely used to treat MDD, there is a need foradditional therapies with a differentiated profile. A potential approval and successful commercialization of the drug will cater to this population.
Both Sage Therapeutics and Biogen are also currently enrolling 300 additional patients in the 50 mg cohort.
Apart from MDD, zuranolone is being evaluated in the NEST clinical program for treating postpartum depression (PPD). Earlier in October, both Sage Therapeutics and Biogen announced their plan to submit a new drug application to the FDA seeking approval for zuranolone to treat MDD in second-half 2022 and PPD in first-half 2023, based on a pre-NDA meeting with the FDA.
Sage Therapeutics, Inc. Price

Sage Therapeutics, Inc. price | Sage Therapeutics, Inc. Quote
Biogen Inc. Price
Biogen Inc. price | Biogen Inc. Quote
Zacks Rank & Stocks to Consider
Both Sage Therapeutics and Biogen currently carry a Zacks Rank #3 (Hold).
Some better-ranked stocks in the overall healthcare sector include Endo International ENDP and GlaxoSmithKline GSK. While Endo International sports a Zacks Rank #1 (Strong Buy), GlaxoSmithKline carries a Zacks Rank #2 (Buy) at present. You can see the complete list of today’s Zacks #1 Rank stocks here.
Endo International’s earnings per share estimates for 2021 have increased from $2.32 to $2.84 in the past 30 days. The same for 2022 has increased from $2.25 to $2.47 in the past 30 days.
Earnings of Endo International beat estimates in all the last four quarters, the average being 57.7%.
GlaxoSmithKline’s earnings estimates per share for 2021 have increased from $2.90 to $3.06 in the past 30 days. The same for 2022 has increased from $3.15 to $3.26 in the past 30 days. Shares of Glaxo have risen 12.4% in the year so far.
Earnings of GlaxoSmithKline beat estimates in three of the last four quarters and missed expectations in one, the average surprise being 15.3%.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
GlaxoSmithKline plc (GSK) : Free Stock Analysis Report
Biogen Inc. (BIIB) : Free Stock Analysis Report
Endo International plc (ENDP) : Free Stock Analysis Report
Sage Therapeutics, Inc. (SAGE) : Free Stock Analysis Report
To read this article on Zacks.com click here.
Zacks Investment Research

 Yahoo Finance
Yahoo Finance 