Theravance's (TBPH) Q2 Earnings Beat, Focus on Pipeline
Theravance Biopharma, Inc. TBPH reported adjusted loss (excluding restructuring and related expenses) was 4 cents per share, narrower than the Zacks Consensus Estimate of a loss of 6 cents and the year-ago quarter’s loss of 80 cents per share.
Total revenues of $11.1 million missed the Zacks Consensus Estimate of $17 million. Revenues declined 14.4% year over year.
Shares of Theravance have declined 14.3% so far this year compared with the industry’s decrease of 19.5%.
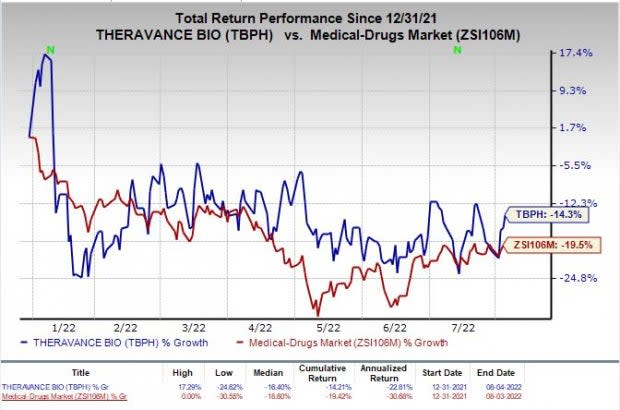
Image Source: Zacks Investment Research
Quarter in Detail
The top line comprised Viatris VTRS collaboration revenues worth $10.9 million in relation to Yupelri (revefenacin).
Theravance and Viatris are developing and commercializing Yupelri, a long-acting muscarinic antagonist, as a once-daily nebulized treatment of chronic obstructive pulmonary disease (COPD). Viatris and Theravance share U.S. profits and losses related to the commercialization of Yupelri. While Viatris gets 65% of the profits, Theravance earns 35%.
Research & development expenses were $15.6 million, down 69.5% from the year-ago quarter’s figure.
Selling, general & administrative expenses were down 34.5% year over year to $17 million.
As of Jun 30, 2022, Theravance had cash, cash equivalents and marketable securities worth $132.9 million compared with $147.5 million as of Mar 31, 2022.
2022 Guidance
Theravance reiterated its financial guidance for 2022. TBPH expects adjusted research & development expenses (excluding one-time restructuring expenses and share-based compensation) in the range of $45-$55 million, while adjusted selling, general and administrative expenses are projected between $35 million and $45 million.
TBPH expects to achieve sustainability in cash flow and become cash flow positive in the second half of 2022.
Other Updates
In July, TBPH entered into a definitive agreement with Royalty Pharma RPRX to sell all of the former’s equity interests in Theravance Respiratory Company LLC for an upfront payment of 1.1 billion. Theravance Respiratory Company, LLC represents 85% economic interest in the sales-based royalty rights on the global net sales of GlaxoSmithKline’s GSK once-daily single inhaler triple therapy for COPD and asthma, Trelegy Ellipta. With the deal in place, royalty interests for GSK’s Trelegy Ellipta now belong to Royalty Pharma. TBPH will also receive up to $250 million in additional milestone payments as a part of the deal. With the deal’s completion, royalty interests for GSK’s Trelegy will belong to Royalty Pharma.
Additionally, Royalty Pharma will be investing $40 million in Theravance to support the clinical development of the latter’s investigational pipeline candidate, ampreloxetine, for treating symptomatic neurogenic orthostatic hypotension (nOH).
In April, Theravance reported data from a phase III study (Study 0170) on ampreloxetine for symptomatic nOH. The study failed to achieve statistical significance in its primary end-point for the overall patient population. However, a sub-group analysis based on the disease type showed that the drug achieved a 72% reduction in the odds of treatment failure with ampreloxetine compared to placebo in multiple system atrophy (MSA) patients.
Based on this data, in June 2022, TBPH held a Type C meeting with the FDA.
The company intends to start a new phase III study by early 2023, evaluating ampreloxetine with a primary endpoint of change in orthostatic hypotension symptom assessment (OHSA) composite score.
Royalty Pharma will invest $25 million to fund a major portion of the study, per the terms of Trelegy deal, and an additional $15 million upon the first regulatory approval of ampreloxetine.
Theravance Biopharma, Inc. Price, Consensus and EPS Surprise
Theravance Biopharma, Inc. price-consensus-eps-surprise-chart | Theravance Biopharma, Inc. Quote
Zacks Rank & Key Pick
Theravance Biopharma currently carries a Zacks Rank #3 (Hold).
You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
GSK PLC Sponsored ADR (GSK) : Free Stock Analysis Report
Royalty Pharma PLC (RPRX) : Free Stock Analysis Report
Theravance Biopharma, Inc. (TBPH) : Free Stock Analysis Report
Viatris Inc. (VTRS) : Free Stock Analysis Report
To read this article on Zacks.com click here.

 Yahoo Finance
Yahoo Finance 