Alnylam (ALNY) Soars as Heart Disease Drug Study Meets Goals
Alnylam Pharmaceuticals ALNY hit a new 52-week high on Monday after it reported positive top-line results from a late-stage study evaluating Amvuttra (vutrisiran), an investigational RNAi therapeutic, for the treatment of transthyretin-mediated (ATTR) amyloidosis with cardiomyopathy. The stock jumped 34.5% in response to the success of the study.
Please note that Amvuttra is already approved in the United States for the treatment of adult patients with polyneuropathy of hereditary ATTR amyloidosis. It is also approved in the EU for the same indication in adult patients with stage 1 or stage 2 polyneuropathy.
The phase III HELIOS-B study, seeking to expand the label of Amvuttra, met the primary endpoint, demonstrating a statistically significant reduction in the composite of all-cause mortality and recurrent cardiovascular events in the overall population as well as the monotherapy population. The monotherapy population comprises those patients who did not receive tafamidis at baseline.
The study also demonstrated statistically significant improvements across all secondary endpoints in both the overall and monotherapy populations. Key measures of disease progression, such as the six-minute walk test, Kansas City Cardiomyopathy Questionnaire and New York Heart Association Class, showed improvements at Month 30.
Additionally, treatment with vutrisiran reduced all-cause mortality in the overall population and the monotherapy population up to Month 42. This was determined through a pre-specified, intent-to-treat analysis that included up to six months of data from an open-label extension.
Moreover, vutrisiran demonstrated consistent effects on the primary composite endpoint and all secondary endpoints across all key subgroups, including baseline tafamidis use, ATTR disease type and measures of disease severity.
Year to date, shares of Alnylam have gained 16.5% against the industry’s 5.9% decline.
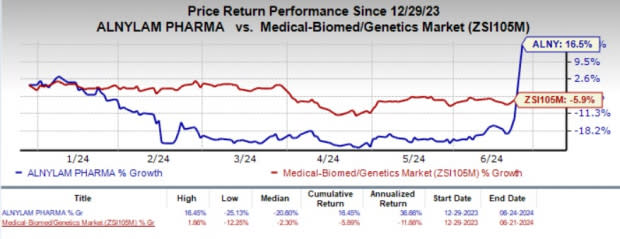
Image Source: Zacks Investment Research
The phase III HELIOS-B study enrolled 655 adult patients with ATTR amyloidosis (hereditary or wild-type) with cardiomyopathy who were divided equally to receive vutrisiran 25mg or placebo subcutaneously once every three months for up to 36 months.
Vutrisiran also demonstrated an encouraging safety and tolerability profile in the late-stage study which was consistent with that observed in previous studies of the drug. The rates of adverse events (AEs), serious AEs, and AEs leading to study drug discontinuation were similar between the vutrisiran and placebo arms. Additionally, no AEs occurred ≥3% more frequently in the vutrisiran arm compared with the placebo arm.
Alnylam is gearing up to proceed with global regulatory submissions starting later in 2024, including filing a supplemental new drug application with the FDA using a Priority Review Voucher.
Subject to approval, Alnylam believes that vutrisiran has the potential to become the new standard of care for the treatment of ATTR amyloidosis with cardiomyopathy. This will expand the eligible patient population for the drug driving substantial growth for the company in the future.
Amvuttra is currently the top contributor to Alnylam’s top line. Amvuttra generated sales worth $195 million in the first quarter, up 92% on a reported basis. The encouraging uptake of the drug is driven by new patients starting treatment as well as patients switching from ALNY’s first-approved drug, Onpattro(patisiran), for treating hereditary ATTR amyloidosis in adults.
Alnylam Pharmaceuticals, Inc. Price and Consensus

Alnylam Pharmaceuticals, Inc. price-consensus-chart | Alnylam Pharmaceuticals, Inc. Quote
Zacks Rank and Stocks to Consider
Alnylam currently carries a Zacks Rank #3 (Hold).
Some better-ranked stocks from the drug/biotech industry are ALX Oncology Holdings ALXO, Annovis Bio ANVS and Compugen CGEN, each carrying a Zacks Rank #2 (Buy) at present. You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
In the past 30 days, the Zacks Consensus Estimate for ALX Oncology’s 2024 loss per share has remained constant at $2.89. During the same period, the consensus estimate for 2025 loss per share has remained constant at $2.73. Year to date, shares of ALXO have plunged 56%.
ALX Oncology beat estimates in two of the trailing four quarters and missed twice, delivering an average negative surprise of 8.83%.
In the past 30 days, the Zacks Consensus Estimate for Annovis’ 2024 loss per share has remained constant at $2.46. During the same period, the consensus estimate for 2025 loss per share has remained constant at $1.95. Year to date, shares of ANVS have plunged 67.2%.
ANVS beat estimates in three of the trailing four quarters and missed once, delivering an average negative surprise of 1.39%.
In the past 30 days, the Zacks Consensus Estimate for Compugen’s 2024 earnings per share has remained constant at 5 cents. The consensus estimate for 2025 loss per share is currently pegged at 11 cents. Year to date, shares of CGEN have lost 5.6%.
CGEN’s earnings beat estimates in three of the trailing four quarters and missed once, delivering an average surprise of 5.79%.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Alnylam Pharmaceuticals, Inc. (ALNY) : Free Stock Analysis Report
Compugen Ltd. (CGEN) : Free Stock Analysis Report
Annovis Bio, Inc. (ANVS) : Free Stock Analysis Report
ALX Oncology Holdings Inc. (ALXO) : Free Stock Analysis Report

 Yahoo Finance
Yahoo Finance 