Bayer (BAYRY) & Cedilla to Co-Develop Novel Cancer Therapies
Bayer BAYRY has announced an exclusive license agreement with Cedilla Therapeutics to jointly develop and commercialize the latter’s CyclinE1/CDK2 complex inhibitors for targeted oncogenic treatments. Cedilla Therapeutics’ CyclinE1/CDK2 complex inhibitors, which are in pre-clinical development, follow a novel mechanism of action that allows selective inhibition of the protein complex in a selected patient population where a high unmet medical need exists.
Per the agreement, BAYRY is set to receive full rights, upon the successful development and commercialization of Cedilla’s CyclinE1/CDK2 complex inhibitors. Financial details regarding the collaboration agreement have not been disclosed. However, Bayer will be liable to make an upfront payment to Cedilla along with milestone payments subject to the achievement of certain development and commercial objectives.
Cedilla is also eligible to receive tiered royalty payments from Bayer on medicines based on the former’s technology commercialized by Bayer.
In the year so far, shares of BAYRY have gained 8.7% against the industry’s 1% decline.
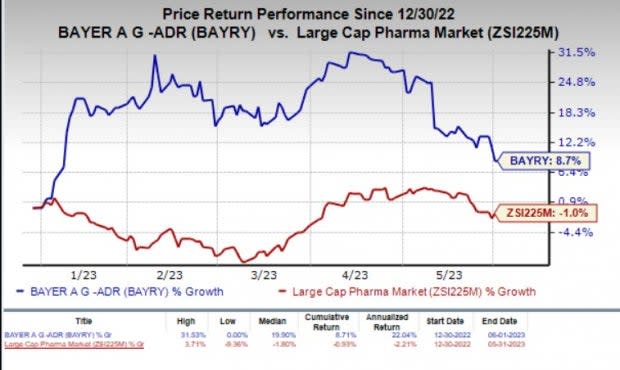
Image Source: Zacks Investment Research
Per Bayer, Cedilla Therapeutics’ proprietary CyclinE1/CDK2 complex inhibitors possess the potential to enhance safety and efficacy in comparison to current standard-of-care treatment options. Management believes that this agreement regarding Cedilla’s preclinical programs will complement its early-stage pipeline in precision oncology. The collaboration will grant Cedilla access to Bayer’s expertise in research and development of novel cancer therapies along with its vast financial resources and commercial network.
In a separate press release, Bayer and partner, Merck MRK, announced the enrollment of the first patient in the phase II/III VALOR study evaluating Verquvo (vericiguat) in pediatric patients (>28 days to 18 years) with heart failure due to left ventricular systolic dysfunction. VALOR is a pivotal study evaluating the efficacy, safety and pharmacokinetics of vericiguat for pediatric heart failure.
In January 2021, Bayer and Merck got approval for Verquvo(vericiguat) to treat adult patients with worsening chronic heart failure with reduced ejection fraction.
The FDA approval for Verquvo was based on data from the phase III VICTORIA study. The VICTORIA study evaluated vericiguat versus placebo when given in combination with available heart failure therapies in patients with symptomatic chronic heart failure and ejection fraction less than 45% following a worsening heart failure event.
Data from the study showed that vericiguat was superior to placebo in reducing the risk of the composite endpoint of heart failure hospitalization or cardiovascular death in such patients. The data showed that there was a 4.2% reduction in annualized absolute risk with Verquvo compared with placebo. Verquvo tablets (2.5 mg, 5 mg and 10 mg) have been jointly developed by Bayer and Merck.
The primary endpoint of the VALOR study is the magnitude of change in plasma concentration of N-terminal pro-brain natriuretic peptide, a diagnostic tool in the diagnosis of heart failure, from baseline to week 16.
Bayer Aktiengesellschaft Price and Consensus
Bayer Aktiengesellschaft price-consensus-chart | Bayer Aktiengesellschaft Quote
Zacks Rank and Stocks to Consider
Bayer currently has a Zacks Rank #3 (Hold).
A couple of better-ranked stocks in the drug/biotech sector are Novartis NVS and Akero Therapeutics AKRO, both carrying a Zacks Rank #2 (Buy) at present. You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
In the past 90 days, the Zacks Consensus Estimate for Novartis’ 2023 earnings per share has increased from $6.52 to $6.67. In the year so far, shares of Novartis have increased by 7%.
NVS beat estimates in each of the trailing four quarters, delivering an average earnings surprise of 5.15%.
In the past 90 days, the Zacks Consensus Estimate for Akero Therapeutics’ 2023 loss per share has narrowed from $3.46 to $2.78. In the year so far, shares of Akero Therapeutics have fallen by 17.7%.
AKRO beat estimates in three of the trailing four quarters, missing the mark on one occasion, delivering an average earnings surprise of 7.96%.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Novartis AG (NVS) : Free Stock Analysis Report
Merck & Co., Inc. (MRK) : Free Stock Analysis Report
Bayer Aktiengesellschaft (BAYRY) : Free Stock Analysis Report
Akero Therapeutics, Inc. (AKRO) : Free Stock Analysis Report

 Yahoo Finance
Yahoo Finance 