Biogen's (BIIB) Lecanemab Shows Benefit but With Side Effects
Biogen BIIB said its partner Eisai presented data from their large phase III confirmatory study, Clarity AD, on anti-amyloid beta protofibril antibody candidate lecanemab (BAN2401) to treat early Alzheimer’s disease (early AD) at the Clinical Trials in Alzheimer’s Congress held in San Francisco.
The Clarity AD study is evaluating lecanemab for the treatment of mild cognitive impairment (MCI) due to AD and mild AD (collectively known as early AD) with the confirmed presence of amyloid pathology in the brain.
The study met the primary endpoint in September. The study’s primary endpoint was Clinical Dementia Rating-Sum of Boxes or CDR-SB, which is a numerical scale that measures the severity of symptoms of dementia. The study met the primary endpoint by showing that treatment with lecanemab in the early stages of the disease reduced the rate of clinical decline on the CDR-SB scale by 27% compared to placebo. The detailed data showed that the mean change of CDR-SB from baseline at 18 months as was 1.21 in the lecanemab arm and 1.66 for the placebo groups.
The study met all key secondary endpoints, demonstrating highly statistically significant results. On a scale called ADAS-Cog14, lecanemab slowed the decline of cognitive function by 26% at 18 months. In another assessment called AD Composite Score (ADCOMS), lecanemab slowed disease progression by 24% at 18 months. In the ADCS MCI-ADL scale, lecanemab slowed the decline of activities of daily living by 37%.
With regard to the candidate’s safety, lecanemab led to infusion-related reactions in 26.4% of the participants and amyloid-related imaging abnormalities (ARIA), a brain swelling side effect associated with anti-amyloid antibodies, with edema or effusions in 12.6% of the participants. Deaths occurred in 0.7% and 0.8% of participants in the lecanemab and placebo groups, respectively. However, Eisai said no deaths were related to lecanemab. Earlier this week, there were reports of the death of a 65-year-old woman from a massive brain hemorrhage, who was given lecanemab in a phase III extension portion.
Such news and adverse events observed in the Clarity AD study raise concerns over the drug’s safety
The detailed data were also published in the New England Journal of Medicine. The NEJM article concluded that the candidate did reduce markers of amyloid in early Alzheimer’s disease and led to moderately less decline in measures of cognition and function than placebo at 18 months. However, treatment with lecanemab was associated with adverse events. The article said that longer trials were needed to establish the candidate’s efficacy and safety in early Alzheimer’s disease.
Biogen’s stock has risen 21.5% this year so far against a decline of 19.9% for the industry. Biogen stock is up more than 49% since the Clarity AD study’s initial data were announced in September
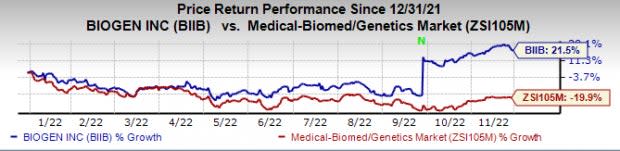
Image Source: Zacks Investment Research
Biogen/Eisai have already filed their biologics license application (“BLA”), seeking accelerated approval for lecanemab with the FDA. The BLA was supported by data from a phase II study (Study 201). The FDA accepted the BLA and granted priority review to the same. The FDA’s decision is expected on Jan 6, 2023.
Eisai plans to file for traditional approval of lecanemab in the United States and submit regulatory applications in the EU and Japan by the end of the first quarter of 2023 based on data from the Clarity AD study
Biogen has developed lecanemab in collaboration with Eisai, with the latter leading the clinical development and regulatory submissions. The companies also developed another anti-amyloid antibody, Aduhelm, which was approved by the FDA in June 2021 but failed to generate meaningful sales.
Some other large-cap pharma giants like Roche RHHBY and Eli Lilly LLY are also developing their candidates targeting the AD indication. The Alzheimer’s candidates of these companies — also anti-amyloid beta antibodies — are in late-stage development or review and are expected to be launched in a few months.
Eli Lilly’s BLA seeking accelerated approval of donanemab, based on data from the TRAILBLAZER-ALZ study, is already under review with the FDA. A decision is expected in early 2023. Lilly also expects a data readout from the phase III TRAILBLAZER-ALZ 2 by mid-2023, which, if positive, will form the basis of its application for traditional regulatory approval for donanemab.
Roche’s phase III GRADUATE I and II studies on key Alzheimer’s pipeline candidate, gantenerumab failed to meet the primary endpointof slowing clinical decline in people with early Alzheimer’s disease earlier this month. In the study, participants treated with gantenerumab showed a slowing of the clinical decline of -0.31 and -0.19, respectively in the GRADUATE I and GRADUATE II studies from the baseline score on the Clinical Dementia Rating-Sum of Boxes scale. However, this decline was not statistically significant in either of the studies. This represented a relative reduction in the clinical decline of 8% in GRADUATE I and 6% in GRADUATE II compared with placebo. The level of beta-amyloid removal by gantenerumab was less than expected. Roche was developing the candidate in collaboration with MorphoSys.
Zacks Rank & Stock to Consider
Biogen has a Zacks Rank #3 (Hold) currently. You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
A better-ranked large drugmaker is Vertex Pharmaceuticals VRTX, which has a Zacks Rank #2 (Buy) at present.
Vertex Pharmaceuticals’ stock has risen 44% this year so far. Estimates for Vertex’s 2022 earnings have gone up from $14.52 to $14.61 per share, while that for 2023 have increased from $15.50 to $15.60 per share over the past 30 days.
Vertex has a four-quarter earnings surprise of 3.16%, on average.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Biogen Inc. (BIIB) : Free Stock Analysis Report
Roche Holding AG (RHHBY) : Free Stock Analysis Report
Eli Lilly and Company (LLY) : Free Stock Analysis Report
Vertex Pharmaceuticals Incorporated (VRTX) : Free Stock Analysis Report

 Yahoo Finance
Yahoo Finance 