FDA Delays Decision on Sanofi (SNY)-Regeneron Dupixent in COPD
Sanofi SNY and Regeneron REGN announced that the FDA extended the review period for a regulatory filing seeking label expansion for their blockbuster drug Dupixent in chronic obstructive pulmonary disease (COPD) indication by an additional three months. A final decision is now expected by Sep 27, 2024.
The FDA filing seeks approval for Dupixent as an add-on maintenance treatment in certain adult patients with uncontrolled COPD.
The delay is on account of the additional data submitted by the companies on the FDA’s request during the ongoing review of Dupixent in COPD. Per the agency, the information submitted by the company constitutes a major amendment to the data filed in its earlier regulatory filing. Management also confirmed that the FDA did not raise any concerns regarding the approvability of the drug in this indication.
The regulatory submission is based on data from the late-stage BOREAS and NOTUS studies, which evaluated Dupixent in adults who were current or former smokers with uncontrolled COPD with type 2 inflammation. Both studies met their primary endpoints, showing that treatment with the drug significantly reduced annualized moderate or severe acute COPD exacerbations by up to 34% compared with placebo. Treatment with Dupixent also rapidly and significantly improved lung function compared to placebo, with improvements sustained at 52 weeks.
Shares of Sanofi and Regeneron were down in pre-market trading on May 31, likely due to the delay in review. Investors were previously expecting approval for Dupixent in COPD indication by the end of next month.
The delayed date is likely to benefit Verona Pharma VRNA, whose regulatory filing is also under FDA review seeking approval for lead drug ensifentrine as a maintenance treatment for patients with COPD. A final decision is expected by Jun 26. If approved, the Verona drug will offer a novel mechanism of action to treat COPD patients. Verona expects to launch the drug this year during the third quarter commercially.
Sanofi’s shares have lost 3.8% in the year against the industry’s 14.6% growth.
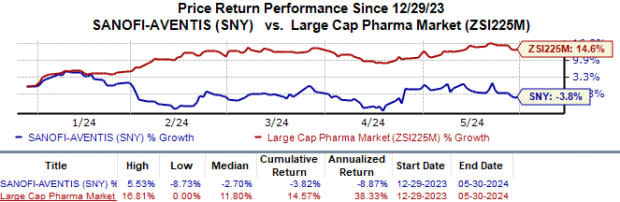
Image Source: Zacks Investment Research
Regeneron’s shares have gained 10.4% year to date against the industry’s 7.1% decline.
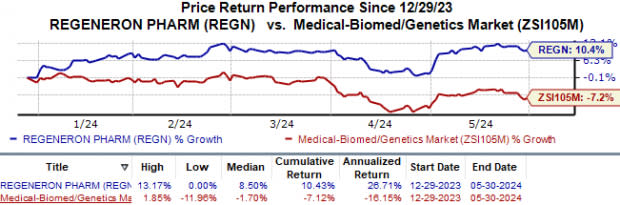
Image Source: Zacks Investment Research
In a separate press release, Regeneron/Sanofi also announced that the EMA’s Committee for Medicinal Products for Human Use (CHMP) issued a positive recommendation on their regulatory filing recommending Dupixent’s label expansion in COPD indication. A final decision on the filing is expected in the coming months. Like the FDA filing, this filing is also supported by data from the BOREAS and NOTUS studies.
COPD is a life-threatening respiratory disease that damages the lungs and causes progressive lung function decline. Smoking is also a key risk factor for COPD. The disease is the third leading cause of death worldwide. Management estimates that around 300,000 people are living with uncontrolled COPD with type II inflammation in the United States. Currently, there are limited treatment options for COPD patients. Per management, no novel treatments have been approved targeting this indication in more than a decade.
An approval for Dupixent in COPD indication will drive the drug’s sales higher.
Dupixent is a key top-line driver for Sanofi and Regeneron. The drug is currently approved in the United States and Europe for five type II inflammatory diseases, namely severe chronic rhinosinusitis with nasal polyposis, severe asthma, moderate-to-severe atopic dermatitis, eosinophilic oesophagitis and prurigo nodularis. With outside U.S. revenues accelerating and multiple approvals for new indications and expansion in younger patient populations expected, its sales are likely to be higher.
During first-quarter 2024, Sanofi recorded €2.84 billion from Dupixent product sales, indicating a 25% year over year growth. Sanofi expects Dupixent to achieve about €13 billion by this year’s end.
Regeneron and Sanofi are jointly marketing Dupixentunder a global collaboration agreement. Sanofi records global net product sales of Dupixent, while Regeneron records its share of profits/losses in connection with the drug’s global sales. Both companies are also studying the drug in late-stage studies in a broad range of diseases driven by type II inflammation, like bullous pemphigoid and chronic pruritus of unknown origin.
Sanofi Price
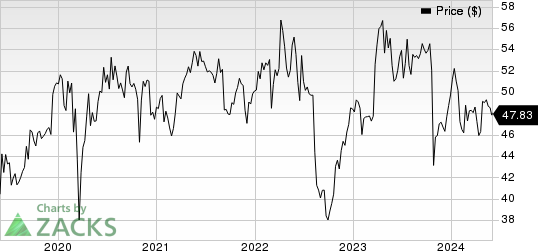
Sanofi price | Sanofi Quote
Regeneron Pharmaceuticals, Inc. Price
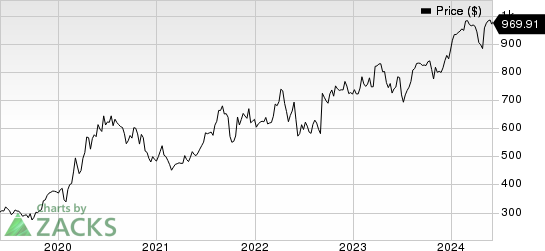
Regeneron Pharmaceuticals, Inc. price | Regeneron Pharmaceuticals, Inc. Quote
Zacks Rank
Sanofi and Regeneron currently carry a Zacks Rank #3 (Hold). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Regeneron Pharmaceuticals, Inc. (REGN) : Free Stock Analysis Report
Sanofi (SNY) : Free Stock Analysis Report
Verona Pharma PLC American Depositary Share (VRNA) : Free Stock Analysis Report

 Yahoo Finance
Yahoo Finance 