Pfizer (PFE) Stock Down 5% as Obesity Drug Fails in Phase II
Pfizer’s PFE shares declined 5.1% on Friday after it announced it will not move forward with phase III studies on its twice-daily weight loss pill, danuglipron, due to side effects seen in a phase IIb study.
The phase IIb study demonstrated a statistically significant change in body weight from baseline, thereby meeting the primary endpoint. Top-line data from the study showed that twice-daily dosing of danuglipron, an oral GLP-1R agonist candidate, resulted in mean placebo-adjusted weight reductions ranging from -8% to -13% at 32 weeks and -5% to -9.5% at 26 weeks.
However, high rates of gastrointestinal side effects were observed in the study, which included up to 73% nausea, up to 47% vomiting and up to 25% diarrhea. Meanwhile, discontinuation rates were greater than 50%, seen across all doses, much higher than approximately 40% with placebo. However, treatment with danuglipron did not lead to any increased incidence of liver enzyme elevation compared to placebo.
The pharmacokinetic study of the once-daily formulation of danuglipron continues. Pfizer, has, for now, decided not to move forward with phase III studies on the twice-daily formulation of danuglipron. Depending on the outcome of the once-daily formulation, it will decide on the path forward.
We remind investors that in June Pfizer had dropped development of another GLP-1-RA candidate, lotiglipron.
Year to date, the stock has plunged 43.6% against the industry’s 4.8% rise.
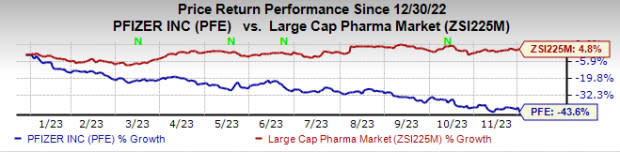
Image Source: Zacks Investment Research
The failure of the phase IIb study on twice-daily oral formulation of danuglipron is a significant blow to Pfizer’s efforts to take a share of the growing market of GLP-1 drugs for treating obesity. GLP-1 obesity drugs are designed in such a way that they suppress appetite so you eat less and eventually lose weight.
The market for obesity and weight management drugs is attracting a lot of interest lately in the United States. Obesity has become a global health problem as it can cause other diseases like heart disease, diabetes and stroke. This has resulted in an exponential increase in demand for these obesity medicines. Also, social media has somewhat hyped the benefits of these medications.
Patients are gradually understanding the benefits of obesity drugs like Novo Nordisk’s NVO popular GLP-1 receptor agonist, Wegovy. Wegovy injection is seeing strong prescription trends and is generating impressive revenues and profits for Novo Nordisk.
Eli Lilly’s LLY tirzepatide, a dual GIP and GLP-1 receptor agonist (GIP/GLP-1 RA), was approved by the name of Zepbound (also an injection) for chronic weight management in the United States in November and should be launched in some weeks.
Novo Nordisk is also evaluating a once-daily oral formulation of Wegovy in late-stage studies. In August, Novo Nordisk announced plans to acquire Canada’s private company Inversago Pharma, which makes CB1 receptor blocker therapies for obesity, diabetes and other serious metabolic diseases. CB1 is a cannabinoid receptor that plays an important role in appetite regulation and other cardiometabolic pathways. Inversago Pharma’s lead pipeline candidate, INV-202, an oral CB1 inverse agonist, demonstrated weight loss potential in an early-stage study.
Lilly’s pipeline also includes retatrutide (GGG tri-agonist) and orforglipron, which are being developed for type II diabetes and obesity. In August, Lilly acquired private biotech Versanis, which is expected to strengthen its position in the obesity market.
Among some other companies, Amgen AMGN has maridebart cafraglutide, also a GLP-1 receptor, in its pipeline that is being studied in a phase II study for treating obesity. Enrollment is complete in the phase II study, with top-line data expected by the end of 2024. Amgen believes maridebart cafraglutide has the potential to result in strong weight loss, both rapid and sustained over time with convenient dosing, which can differentiate it from what is available in the market now.
Zacks Rank & Stock to Consider
Currently, Pfizer has a Zacks Rank #3 (Hold). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Pfizer Inc. Price and Consensus
Pfizer Inc. price-consensus-chart | Pfizer Inc. Quote
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Pfizer Inc. (PFE) : Free Stock Analysis Report
Novo Nordisk A/S (NVO) : Free Stock Analysis Report
Eli Lilly and Company (LLY) : Free Stock Analysis Report
Amgen Inc. (AMGN) : Free Stock Analysis Report

 Yahoo Finance
Yahoo Finance 