Roche's (RHHBY) Ocrevus Subcutaneous Gets European Commission Nod
Roche RHHBY announced that the European Commission has granted marketing authorization to the subcutaneous (SC) formulation of relapsing multiple sclerosis (RMS) and primary progressive multiple sclerosis (PPMS) drug Ocrevus (ocrelizumab).
The approval is based on pivotal data from the late-stage OCARINA II trial, which showed non-inferior levels of the drug in the blood when administered subcutaneously, and a safety and efficacy profile comparable to the intravenous (IV) formulation in patients with RMS and PPMS.
The SC formulation of Ocrevus was developed to provide an alternative twice-a-year treatment option to patients and healthcare professionals, in addition to IV.
Ocrevus SC combines Ocrevus with Halozyme Therapeutics’ ENHANZE drug delivery technology.
Roche also announced that the European Medicines Agency (“EMA”) has validated and initiated review of the marketing authorization application (MAA) for Elevidys (delandistrogene moxeparvovec).Elevidys, a gene therapy for the treatment of ambulatory patients aged 3-7 years with Duchenne muscular dystrophy, is already approved in the United States and some other countries. It is a one-time treatment administered through a single IV dose.
The MAA is based on results from the phase III EMBARK study, a global, randomized, double-blind, placebo-controlled study in DMD patients aged 4 through 7 years. Although the EMBARK study did not meet the primary endpoint, the totality of evidence from the trial confirmed that Elevidys is the first gene therapy to provide clinically meaningful benefits for patients by modifying the disease course, with a manageable safety profile.
Elevidys-treated patients reported clinically meaningful and statistically significant benefits in both key secondary functional endpoints — time to rise from the floor and a ten-minute walk/run test.
In addition, a clinically meaningful and statistically significant improvement was observed for the pre-specified secondary endpoint — stride velocity 95th centile.
The MAA is also supported by data from another study in DMD patients, ENDEAVOR, an open-label study that is enrolling ambulatory and non-ambulatory patients of various ages, along with a phase I/II study which provides long-term efficacy, durability, safety and biological data to support the benefit-risk assessment.
Per Roche, a potential approval will make Elevidys the first and only gene therapy to address the underlying cause of DMD, available in Europe.
Two other studies are also currently ongoing to expand the drug’s label ENVOL, a phase II study in boys with DMD under the age of 4 and ENVISION, a phase III study in older ambulatory and non-ambulatory patients with DMD.
Roche has a collaboration agreement with Sarepta Therapeutics SRPT for Elevidys, whereby it is responsible to commercialize Elevidys outside the United States.
Last week, Sarepta announced that the FDA has converted the accelerated approval granted to Elevidys to a traditional approval and expanded the label to include ambulatory individuals with a confirmed mutation in the DMD gene who are aged four and older. In addition, the FDA also granted accelerated approval to Elevidys for non-ambulatory Duchenne patients.
Roche’s shares have lost 1.4% year to date against the industry’s growth of 22.1%.
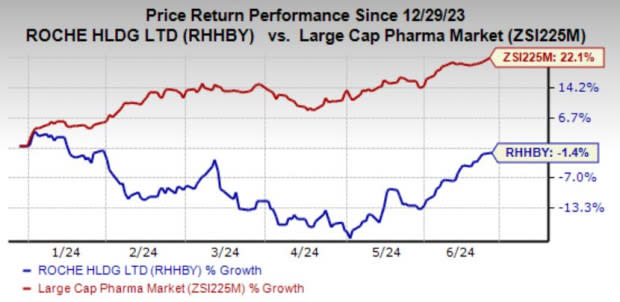
Image Source: Zacks Investment Research
Approval of new drugs and label expansion of the existing ones should bode well for Roche in this scenario.
Drugs like Vabysmo, Ocrevus, Hemlibra and Polivy fuel Roche’s top line as the company looks to fill up the dent in revenues caused by a decline in COVID-19-related sales. Competition from biosimilars for established drugs like Avastin, MabThera/Rituxan and Herceptin continues to hurt sales.
Zacks Rank & Other Stocks to Consider
Roche currently carries a Zacks Rank #2 (Buy).
A couple of other top-ranked stocks in the healthcare sector are ALX Oncology Holdings ALXO and Minerva Neurosciences, Inc. NERV, each carrying a Zacks Rank #2 at present. You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
In the past 60 days, the Zacks Consensus Estimate for ALX Oncology’s 2024 loss per share has narrowed from $3.33 to $2.89. During the same period, the consensus estimate for 2025 loss per share has narrowed from $2.85 to $2.73.
ALX Oncology beat on earnings in two of the trailing four quarters and missed the mark in the other two, delivering an average negative surprise of 8.83%.
In the past 60 days, estimates for Minerva Neurosciences’ 2024 loss per share have narrowed from $3.57 to $1.89. The loss per share estimate for 2025 has narrowed from $4.54 to $3.60 during the same time frame.
NERV’s earnings beat estimates in one of the trailing four quarters and missed the same in the other three, the average negative surprise being 54.43%.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Roche Holding AG (RHHBY) : Free Stock Analysis Report
Sarepta Therapeutics, Inc. (SRPT) : Free Stock Analysis Report
Minerva Neurosciences, Inc (NERV) : Free Stock Analysis Report
ALX Oncology Holdings Inc. (ALXO) : Free Stock Analysis Report

 Yahoo Finance
Yahoo Finance 