AstraZeneca (AZN) Falls on Failure to Meet NSCLC Study OS Goal
AstraZeneca AZN reported overall survival (OS) data from a late-stage study, which evaluated its partner Daiichi Sankyo’s investigational candidate, datopotamab deruxtecan (Dato-DXd), compared with docetaxel in patients with locally advanced or metastatic non-small cell lung cancer (NSCLC). Docetaxel is the current standard-of-care chemotherapy for this patient population.
Though the OS results numerically favored Dato-DXd, it failed to achieve statistical significance in the overall population of the phase III TROPION-Lung01 study. Shares of AstraZeneca declined 2.4% on May 28 in response to the news.
Dato-DXd is a specifically engineered TROP2-directed DXd antibody-drug conjugate discovered by Daiichi Sankyo. The drug is being jointly developed by AstraZeneca and Daiichi Sankyo.
However, in the prespecified subgroup of patients with non-squamous NSCLC, a clinically meaningful improvement in OS upon treatment with Dato-DXd was observed compared with docetaxel.
Please note that OS is one of the dual primary endpoints of the phase III TROPION-Lung01 study evaluating Dato-DXd in previously treated NSCLC patients. The other co-primary endpoint of the late-stage study is progression-free survival (PFS).
We remind the investors that in October 2023, AstraZeneca reported achieving statistically significant improvement in PFS in the TROPION-Lung01 study upon treatment with Dato-DXd compared with docetaxel in the overall NSCLC patient population of the TROPION-Lung01 study.
In non-squamous NSCLC patients, treatment with Dato-DXd also resulted in a clinically meaningful PFS benefit compared with docetaxel.
Year to date, shares of AZN have gained 13.8% compared with the industry’s 15.9% growth.
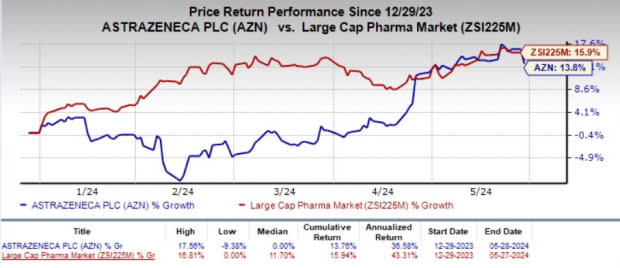
Image Source: Zacks Investment Research
In the press release, the company also stated that Dato-DXd demonstrated a favorable safety profile in the phase III TROPION-Lung01 study, which was consistent with that observed in previous studies. There were fewer dose reductions or discontinuations due to adverse events upon treatment with the candidate than with docetaxel.
Additionally, no new safety concerns or drug-related interstitial lung disease events of any grade were identified.
AstraZeneca and Daiichi Sankyo have already filed a biologics license application (BLA) seeking approval of Dato-DXd for advanced nonsquamous NSCLC based on PFS data from the TROPION-Lung01 study in the United States. The FDA’s decision on the BLA is expected in the fourth quarter of 2024. Regulatory applications are also under review in other countries, including the EU.
AstraZeneca is also simultaneously evaluating Dato-DXd in earlier lines of NSCLC treatment in separate ongoing clinical studies.
An application seeking approval for Dato-DXd for the treatment of HR+ HER2- breast cancer is also currently under review in the United States. A final decision from the FDA for this indication is expected in the first quarter of 2025.
AstraZeneca PLC Price and Consensus
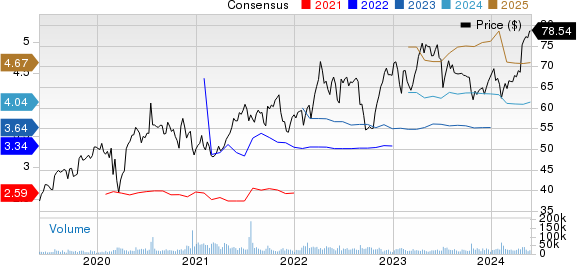
AstraZeneca PLC price-consensus-chart | AstraZeneca PLC Quote
Zacks Rank and Stocks to Consider
AstraZeneca currently carries a Zacks Rank #3 (Hold).
Some better-ranked stocks from the drug/biotech industry are ALX Oncology Holdings ALXO, Annovis Bio ANVS and Entera Bio Ltd. ENTX, each carrying a Zacks Rank #2 (Buy) at present. You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
In the past 30 days, the Zacks Consensus Estimate for ALX Oncology’s 2024 loss per share has narrowed from $3.33 to $2.89. During the same period, the consensus estimate for 2025 loss per share has narrowed from $2.85 to $2.73. Year to date, shares of ALXO have lost 15.6%.
ALX Oncology beat estimates in two of the trailing four quarters and missed twice, delivering an average negative surprise of 8.83%.
In the past 30 days, the Zacks Consensus Estimate for Annovis’ 2024 loss per share has narrowed from $3.35 to $2.46. During the same period, the consensus estimate for 2025 loss per share has narrowed from $2.82 to $1.95. Year to date, shares of ANVS have plunged 64.4%.
ANVS beat estimates in three of the trailing four quarters and missed once, delivering an average negative surprise of 1.39%.
In the past 30 days, the Zacks Consensus Estimate for Entera Bio’s 2024 loss per share has remained constant at 25 cents. During the same period, the consensus estimate for 2025 loss per share has remained constant at 54 cents. Year to date, shares of ENTX have skyrocketed 275%.
ENTX’s earnings beat estimates in three of the trailing four quarters and missed once, delivering an average surprise of 6.50%.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
AstraZeneca PLC (AZN) : Free Stock Analysis Report
Entera Bio Ltd. (ENTX) : Free Stock Analysis Report
Annovis Bio, Inc. (ANVS) : Free Stock Analysis Report
ALX Oncology Holdings Inc. (ALXO) : Free Stock Analysis Report

 Yahoo Finance
Yahoo Finance 