Vertex (VRTX) Begins Rolling NDA Filing for Acute Pain Drug
Vertex Pharmaceuticals Incorporated VRTX announced that the FDA has granted a rolling new drug application (NDA) submission for suzetrigine (formerly known as VX-548) in moderate-to-severe acute pain. The company has already initiated the rolling submission process and is on track to complete the NDA submission in the second quarter of 2024.
Suzetrigine is an investigational orally administered selective NaV1.8 pain signal inhibitor. The rolling NDA submission is supported by encouraging results from the company’s phase III program for suzetrigine in acute pain management.
In January 2024, Vertex reported that it had met the primary endpoints in three phase III studies evaluating suzetrigine to treat moderate-to-severe acute pain. The late-stage studies included two pivotal phase III acute pain studies, one following bunionectomy surgery and the other following abdominoplasty surgery, and a 14-day single-arm phase III study across a broad range of other surgical and non-surgical acute pain conditions.
Data from the studies showed that treatment with suzetrigine led to a significant reduction in pain intensity across a range of pain conditions, both surgical and non-surgical, and in various settings. Suzetrigine enjoys the FDA’s Fast Track and Breakthrough Therapy designations in the United States for moderate-to-severe acute pain.
Apart from the progress made in the acute pain indication, Vertex has also shared updates from the suzetrigine developmental program for neuropathic pain.
We remind the investors that in December 2023, Vertex reported positive data from a mid-stage study on VX-548 in painful diabetic peripheral neuropathy (DPN), a form of peripheral neuropathic pain caused by damage to nerves. Treatment with the drug showed a statistically significant and clinically meaningful reduction in pain intensity.
Following the successful phase II data readout, the company held discussions with the FDA to figure out a path forward for the investigational candidate in DPN. Following dialogues, Vertex is currently gearing up to initiate a pivotal phase III program of suzetrigine in patients with DPN in the second half of 2024.
Notably, suzetrigine also enjoys the FDA’s Breakthrough Therapy designation in the United States for the treatment of pain associated with DPN.
Year to date, shares of Vertex have lost 3.3% compared with the industry’s 10.4% decline.
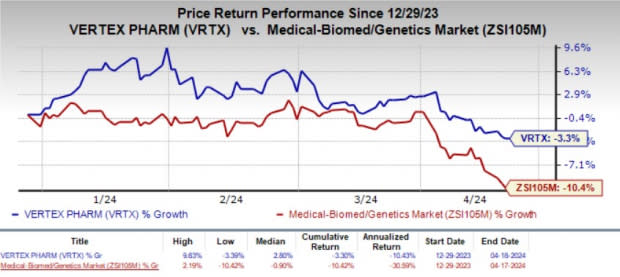
Image Source: Zacks Investment Research
The planned pivotal late-stage program for suzetrigine will include two identical 12-week randomized studies evaluating the efficacy and safety of suzetrigine (70 mg once daily) compared with placebo in patients with DPN. The phase III studies are expected to enroll approximately 1,100 DPN patients each.
Furthermore, both late-stage studies will have a common primary endpoint, i.e. change from baseline in the weekly average of daily pain intensity on the numeric pain rating scale (NPRS) assessed at week 12 compared with placebo. The common secondary endpoint of both studies will be the change from baseline in the weekly average of daily pain intensity on the NPRS at week 12 compared with Pfizer’s Lyrica (pregabalin).
Patients who complete the course of treatment with suzetrigine in the late-stage pivotal studies will have the option to roll over into an open-label study to evaluate the long-term safety and effectiveness of suzetrigine in DPN.
The company is also evaluating suzetrigine in a phase II study in patients with painful lumbosacral radiculopathy, another form of peripheral neuropathic pain. Enrollment in the mid-stage study is currently ongoing and is on track to be completed by the end of 2024.
Vertex believes that VX-548 has potential as it can change the standard of care for neuropathic pain, an area with limited treatment options, mostly highly addictive opioid-based medications.
Besides the clinical program for suzetrigine, VRTX is also developing several other preclinical and clinical NaV1.8 and NaV1.7 pain signal inhibitors, either as a monotherapy or in combination for treating acute and neuropathic pain.
Vertex is preparing to initiate phase II studies on its next-generation NaV1.8 pain signal inhibitor, VX-993 oral formulation, for acute pain and peripheral neuropathic pain later in 2024. The company also expects to initiate a phase I study of an intravenous formulation of VX-993 in 2024.
Vertex Pharmaceuticals Incorporated Price and Consensus
Vertex Pharmaceuticals Incorporated price-consensus-chart | Vertex Pharmaceuticals Incorporated Quote
Zacks Rank & Stocks to Consider
Vertex currently carries a Zacks Rank #3 (Hold).
Some better-ranked stocks from the drug/biotech industry are ADMA Biologics ADMA, FibroGen FGEN and Annovis Bio ANVS. While ADMA sports a Zacks Rank #1 (Strong Buy), FGEN and ANVS carry a Zacks Rank #2 (Buy) each at present. You can see the complete list of today’s Zacks #1 Rank stocks here.
In the past 30 days, the Zacks Consensus Estimate for ADMA Biologics’ 2024 earnings per share (EPS) has remained constant at 30 cents. During the same period, the estimate for ADMA’s 2025 EPS has remained constant at 50 cents. Year to date, shares of ADMA have soared 37.2%.
ADMA beat estimates in three of the trailing four quarters and matched in one, delivering an average earnings surprise of 85%.
In the past 30 days, the Zacks Consensus Estimate for FibroGen’s 2024 loss per share has remained constant at $1.09. During the same period, the estimate for FibroGen’s 2025 loss per share has remained constant at 6 cents. Year to date, shares of FGEN have rallied 32%.
FGEN beat estimates in two of the trailing four quarters, missing the mark on the other two occasions, delivering an average negative surprise of 2.26%.
In the past 30 days, the Zacks Consensus Estimate for Annovis’ 2024 loss per share has narrowed from $3.49 to $3.35. The estimate for Annovis’ 2025 loss per share is currently pegged at $2.82. Year to date, shares of ANVS have plunged 47.3%.
ANVS’ beat estimates in two of the trailing four quarters and missed the mark on the other two occasions, delivering an average negative surprise of 15.70%.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Vertex Pharmaceuticals Incorporated (VRTX) : Free Stock Analysis Report
ADMA Biologics Inc (ADMA) : Free Stock Analysis Report
FibroGen, Inc (FGEN) : Free Stock Analysis Report
Annovis Bio, Inc. (ANVS) : Free Stock Analysis Report

 Yahoo Finance
Yahoo Finance 