Biotech Stock Roundup: IRWD Announces Buyout, ICPT, SRPT & PTCT Fall on Updates
The biotech sector has been in focus in the past week with key pipeline and regulatory updates. Among these, Intercept Pharmaceuticals ICPT was down significantly after a disappointing review for its promising non-alcoholic steatohepatitis (NASH) treatment. Meanwhile, mergers and acquisitions are back in focus.
Recap of the Week’s Most Important Stories:
Intercept Down Post Advisory Meeting: Intercept Pharmaceuticals’ shares plunged after the FDA’s Gastrointestinal Drugs Advisory Committee (“GIDAC”) Meeting voted against the approval of the company’s new drug application (NDA) for obeticholic acid (OCA) for the treatment of pre-cirrhotic fibrosis due to NASH.
Twelve of the 16 voting-eligible advisors voted negative on the question, “given the available efficacy and safety data, do the benefits of OCA 25 mg outweigh the risks in NASH patients with stage 2 or 3 fibrosis?” Fifteen of 16 voting-eligible GIDAC members (with no abstentions) voted to “defer approval until clinical outcome data from trial 747-303 are submitted and reviewed, at which time the traditional approval pathway could be considered.” The outcome of the meeting will provide insights to the FDA, which is scheduled to decide on the NDA on Jun 22, 2023. However, the FDA is not bound by this outcome.
Shares of Intercept were down last week after the FDA publicly posted briefing documents in advance of this meeting due to concerns about drug-induced liver injury. The document stated that, during the review, the FDA identified modest benefits and serious risks of OCA for treating NASH.
Intercept currently has a Zacks Rank #3 (Hold). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Ironwood Gains on News of VectivBio Buyout: Shares of Ironwood Pharmaceuticals, Inc. IRWD gained after it announced that it will acquire clinical-stage biopharmaceutical company VectivBio Holding AG VECT for $17.00 per share in an all-cash transaction for approximately $1 billion. The acquisition will add VectivBio’s lead candidate, apraglutide, for treating short-bowel syndrome patients with intestinal failure to Ironwood’s pipeline. The candidate is in phase III clinical study with plans for a top-line readout by the end of 2023. Ironwood aims to strengthen its pipeline for gastrointestinal diseases with the acquisition. Ironwood’s portfolio includes Linzess, a widely used and successful gastrointestinal treatment. The agreement has been approved by both companies' boards of directors and is expected to be closed in the second half of 2023, subject to customary closing conditions. Shares of VectivBio also surged on the same.
Sarepta Down on Regulatory Update: Shares of Sarepta SRPT declined following updates on the company’s biologics license application (BLA) seeking accelerated approval for SRP-9001 to treat Duchenne muscular dystrophy (DMD). Sarepta stated that the FDA has indicated that the regulatory body will potentially grant accelerated approval for SRP-9001, initially for use in Duchenne patients aged four-five years old only. The agency has stated that it will grant a non-age restricted expansion to SRP-9001 provided the phase III EMBARK study achieves its objectives. EMBARK is the proposed confirmatory study seeking full approval for SRP-9001 in DMD indication. Top-line results from the EMBARK study are expected in fourth-quarter 2023.
The FDA also needs additional time to complete the BLA review, including final label negotiations and post-marketing commitment discussions. As a result, the agency’s final decision on the BLA is now expected before Jun 22, 2023. The agency had previously planned to complete the SRP-9001 review by May 29. The delay disappointed investors.
PTC Therapeutics Down on Study Failure: Shares of PTC Therapeutics PTCT have been on a downward trajectory this week after the company announced that the late-stage MOVE-FA study, evaluating vatiquinone in patients with Friedreich ataxia (FA) failed to achieve its primary endpoint of a significant change in modified Friedreich Ataxia Rating Scale (mFARS) score. The MOVE-FA study failed to achieve statistically significant change in mFARS score at 72 weeks in the primary analysis population. The mFARS is a clinical assessment that measures disease progression, namely swallowing and speech, upper and lower limb coordination and upright stability.
However, treatment with vatiquinone did demonstrate significant benefits on key disease subscales and secondary endpoints.
PTC Therapeutics also announced that it has decided to discontinue pre-clinical and early research programs in gene therapy. The discontinued programs include pre-clinical stage programs in FA and Angelman syndrome as well as several other programs targeting rare CNS and ophthalmological disorders of high unmet medical need. However, PTCT will continue its development and global commercial activities on Upstaza, a gene therapy approved in Europe for treating aromatic L-amino acid decarboxylase (AADC) deficiency in patients aged 18 months and older.
Performance
The Nasdaq Biotechnology Index has lost 0.22% in the past five trading sessions. Among the biotech giants, Moderna has jumped 4.79% during the period. Over the past six months, shares of MRNA have declined 25.4%. (See the last biotech stock roundup here: Biotech Stock Roundup: BIIB’s Q1 Results, ARWR Up on Data, INCY & BLUE Offer Update)
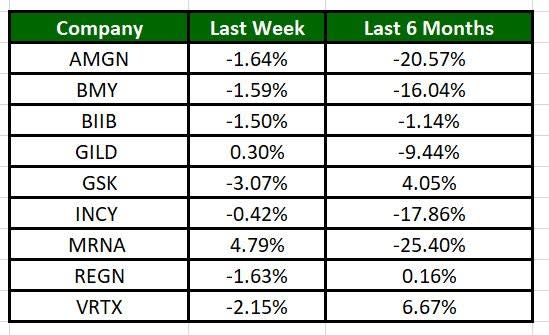
Image Source: Zacks Investment Research
What's Next in Biotech?
Stay tuned for more earnings and pipeline updates.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Ironwood Pharmaceuticals, Inc. (IRWD) : Free Stock Analysis Report
Sarepta Therapeutics, Inc. (SRPT) : Free Stock Analysis Report
PTC Therapeutics, Inc. (PTCT) : Free Stock Analysis Report
Intercept Pharmaceuticals, Inc. (ICPT) : Free Stock Analysis Report
VectivBio Holding AG (VECT) : Free Stock Analysis Report

 Yahoo Finance
Yahoo Finance 