Biotech Stock Roundup: NVAX, FULC Up on Deals With SNY, Updates From MRNA, BMY
As the earnings come to an end in the ongoing reporting cycle, the focus shifts to pipeline and regulatory updates. Collaborations and agreements continue to be in the spotlight as pharma and biotech majors look to bolster their product portfolio/pipeline.
Recap of the Week’s Most Important Stories:
NVAX Surges on Sanofi Deal: Shares of Novavax, Inc. NVAX skyrocketed after the company entered into a co-exclusive licensing agreement with Sanofi SNY for up to $1.2 billion. Per the terms, Sanofi will gain rights to co-market Nuvaxovid globally from next year, except in certain countries where Novavax has existing partnership agreements. SNY also gets the sole license to develop and market the Novavax vaccine in combination with its influenza vaccine.
In exchange, Novavax will get an upfront payment of $500 million and up to $700 million in development, regulatory and launch milestones. Additionally, Novavax is eligible to receive tiered double-digit percentage royalty payments on sales by Sanofi of Nuvaxovid, its influenza-COVID combination vaccine and any other combination vaccines that Sanofi may develop, including Nuvaxovid. Both companies will still have the right to create their own combined COVID-19-influenza combination vaccines and adjuvanted products at their own cost.
Sanofi will also have a non-exclusive license to use the company’s Matrix-M adjuvant technology in other vaccine products. In return, Novavax is entitled to receive a milestone payment of up to $200 million from Sanofi, plus mid-single-digit royalties. Sanofi will also make a minority (<5%) equity investment in Novavax.
The deal overshadowed Novavax’s first-quarter results. Novavax reported a first-quarter 2024 loss of $1.05 per share, wider than the Zacks Consensus Estimate of a loss of $1.04. In the year-ago quarter, the company reported a loss of $3.41. Revenues in the quarter amounted to $94 million, up 16% on a year-over-year basis.
Fulcrum Up on Sanofi Collaboration: Shares of clinical-stage biopharmaceutical company Fulcrum Therapeutics, Inc. FULC, appreciated after the company entered into a collaboration and license agreement with pharma bigwig Sanofi. Per the agreement, Sanofi obtains exclusive commercialization rights for losmapimod, an oral small molecule being investigated for treating facioscapulohumeral muscular dystrophy (FSHD) outside of the United States. The candidate is currently being evaluated in a late-stage study for the treatment of FSHD. This chronic and progressive genetic muscular disorder is characterized by significant muscle cell death and fat infiltration into muscle tissue.
In lieu, Fulcrum will receive an upfront payment of $80 million and is eligible to receive up to an additional $975 million in specified regulatory and sales-based milestones, along with tiered escalating royalties starting in the low-teens on annual net sales of losmapimod outside the U.S. Fulcrum and Sanofi will equally share future global development costs. FULC retains full commercialization rights for the candidate in the United States.
Setback For MRNA: Moderna, Inc. MRNA announced that the FDA delayed the review timeline for its mRNA-based, respiratory syncytial virus (RSV) vaccine, mRNA-1345. Moderna developed mRNA-1345 for use in older adults (aged 60 years and above).
The regulatory body notified the company that due to administrative constraints, it would not be able to complete the review of the biologics license application (BLA) for mRNA-1345 by the Prescription Drug User Fee Act date of May 12, 2024. The FDA extended the review time for the above BLA by a couple of weeks. The review is now expected to be completed by the end of this month.
The FDA, however, did not identify any issues related to the safety, efficacy or quality of mRNA-1345, which could have delayed the approval of the vaccine. Moderna believes that the extended review timeline for mRNA-1345 is not likely to affect the launch plans for the vaccine. The Centers for Disease Control and Prevention (CDC) Advisory Committee on Immunization Practices is expected to review mRNA-1345 in its meeting to be held in June 2024, which is a necessity before the commercial launch of the vaccine.
Updates from BMY: Bristol Myers BMY announced that the FDA had granted accelerated approval to the label expansion of chimeric antigen receptor (CAR) T cell therapy Breyanzi (lisocabtagene maraleucel; liso-cel). The regulatory body approved Breyanzi for the treatment of adult patients with relapsed or refractory follicular lymphoma who have received two or more prior lines of systemic therapy. This indication is approved under accelerated approval based on response rate and duration of response.
Breyanzi is approved in the United States for the treatment of relapsed or refractory large B-cell lymphoma after at least one prior line of therapy. The therapy was also granted accelerated approval for the treatment of relapsed or refractory chronic lymphocytic leukemia or small lymphocytic lymphoma after at least two prior lines of therapy.
BMY also announced that the late-stage CheckMate -73L trial did not meet its primary endpoint of progression-free survival (PFS) in unresectable, locally advanced stage III non-small cell lung cancer (NSCLC). The study evaluated Opdivo (nivolumab) with concurrent chemoradiotherapy (CCRT) followed by Opdivo plus Yervoy (ipilimumab) compared to CCRT followed by durvalumab in patients with unresectable stage III NSCLC.
Performance
The Nasdaq Biotechnology Index has moved up 2.32% in the past five trading sessions and Biogen’s shares have risen 7.1% during the same time frame. In the past six months, shares of MRNA have rallied 69.04%. (See the last biotech stock roundup here: Biotech Stock Roundup: GILD’s GSK’ Q1 Earnings, DCPH Soars on Acquisition News & More)
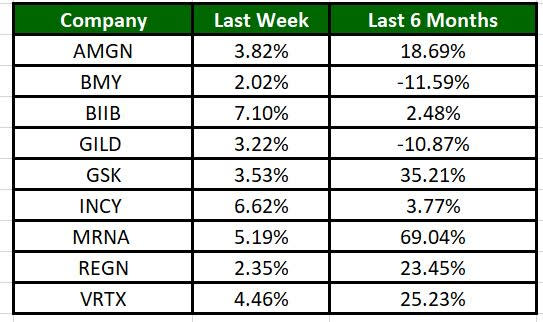
Image Source: Zacks Investment Research
What's Next in Biotech?
Stay tuned for more earnings and pipeline updates.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Sanofi (SNY) : Free Stock Analysis Report
Bristol Myers Squibb Company (BMY) : Free Stock Analysis Report
Moderna, Inc. (MRNA) : Free Stock Analysis Report
Novavax, Inc. (NVAX) : Free Stock Analysis Report
Fulcrum Therapeutics, Inc. (FULC) : Free Stock Analysis Report

 Yahoo Finance
Yahoo Finance 