Capricor (CAPR) Advances in DMD Therapy With Positive Study Data
Capricor Therapeutics CAPR, a biotechnology firm focused on innovative therapies for rare diseases, has announced favorable three-year results from the ongoing HOPE-2 open-label extension (OLE) study on CAP-1002 for Duchenne muscular dystrophy (DMD).
Patients treated with CAP-1002 showed sustained improvements in upper limb function and cardiac performance compared to an external dataset from Cincinnati Children’s Hospital Medical Center, indicating potential long-term benefits.
Statistical Significance in Disease Attenuation
The HOPE-2 OLE study revealed a statistically significant reduction in the decline of upper limb function (PUL 2.0) and stabilization of left ventricular ejection fraction among CAP-1002-treated patients over three years. This contrasts sharply with the expected decline observed in untreated DMD patients, suggesting that CAP-1002 can potentially slow disease progression and improve quality of life.
CAP-1002 has demonstrated a well-tolerated safety profile throughout the study. This favorable safety data supports the continued use of CAP-1002 in treating DMD and provides reassurance for patients and healthcare providers about the long-term use of this therapeutic approach.
Strategic Pathway to FDA Approval
Capricor highlighted the significance of these findings in a recent Type-B meeting with the FDA, which could expedite the pathway to the approval of a Biologics License Application (BLA) and commercialization. The company anticipates announcing top-line results from the Phase 3 HOPE-3 pivotal trial by the fourth quarter of 2024.
With continued progress and regulatory engagement, CAP-1002 may soon offer a much-needed therapeutic option for patients battling this debilitating disease.
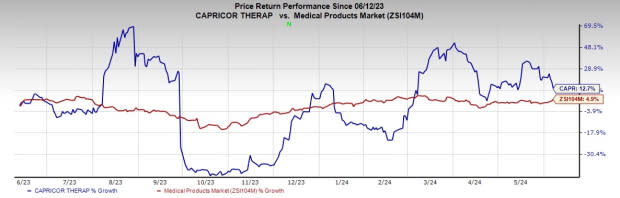
Image Source: Zacks Investment Research
Growing Market Prospects for DMD Treatment
DMD is a serious genetic disorder showing progressive weakness and chronic inflammation of the skeletal, heart and respiratory muscles with mortality at a median age of approximately 30 years. Per Capricor, DMD occurs in approximately one in every 3,500 male births and the patient population is estimated to be approximately 15,000-20,000 alone in the United States.
Going by a Mordor Intelligence report, the DMD treatment market size is estimated at $3.42 billion in 2024, and is expected to reach $8.19 billion by 2029, at a CAGR of 19.1%.
The latest development by Capricor accordingly seems to be a strategic fit and well-timed.
Share Price Performance
Over the past year, shares of CAPR have risen 12.7% compared with the industry’s 4.9% growth.
Zacks Rank & Key Picks
Capricor currently carries a Zacks Rank #4 (Sell).
Some better-ranked stocks in the broader medical space are Hims & Hers Health HIMS, Medpace MEDP and ResMed RMD, each sporting a Zacks Rank #1 (Strong Buy) currently. You can see the complete list of today’s Zacks Rank #1 stocks here.
Hims & Hers Heath stock has surged 131.3% in the past year. Estimates for the company’s earnings have remained constant at 18 cents for 2024 and increased 3.1% to 33 cents for 2025 in the past 30 days.
HIMS’ earnings beat estimates in three of the trailing four quarters and missed in one, delivering an average surprise of 79.2%. In the last reported quarter, it posted an earnings surprise of a staggering 150%.
Estimates for Medpace’s 2024 earnings per share have moved up to $11.29 from $11.23 in the past 30 days. Shares of the company have surged 84% in the past year compared with the industry’s 4.7% growth.
MEDP’s earnings surpassed estimates in each of the trailing four quarters, the average surprise being 12.8%. In the last reported quarter, it delivered an earnings surprise of 30.6%.
Estimates for ResMed’s fiscal 2024 earnings per share have moved to $7.70 from $7.64 in the past 30 days. Shares of the company have declined 1.8% in the past year against the industry’s rise of 4.9%.
RMD’s earnings surpassed estimates in three of the trailing four quarters and missed in one, the average surprise being 2.8%. In the last reported quarter, it delivered an earnings surprise of 10.9%.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
ResMed Inc. (RMD) : Free Stock Analysis Report
Capricor Therapeutics, Inc. (CAPR) : Free Stock Analysis Report
Medpace Holdings, Inc. (MEDP) : Free Stock Analysis Report
Hims & Hers Health, Inc. (HIMS) : Free Stock Analysis Report

 Yahoo Finance
Yahoo Finance 