Catalent (CTLT) Launches Expanded OneBio Suite to Aid Customers
Catalent, Inc. CTLT recently announced the expansion of its integrated development, manufacturing and supply solution, OneBio Suite, across a range of biologic modalities. This includes antibody and recombinant proteins, cell and gene therapies and mRNA.
The OneBio Suite was originally launched in 2019 for early-phase protein therapy development. It offers customers an integrated service to accelerate programs from development to manufacturing, including fill/finish and packaging, and support for clinical supply and commercial launch.
The latest expansion to its offering is expected to significantly boost its BioModalities (Cell, Gene and Protein Therapies) business on a global scale and solidify its foothold in the niche space.
Significance of the Latest Expansion
The newly expanded OneBio will continue to provide integrated contract and proposal, harmonized project management, and a global development and manufacturing network, which will now be extended to biologic modalities and service offerings. Additionally, Catalent’s OneBio offering will include multi-modality, turn-key platforms aimed to speed up clinical trial supply under the UpTempo brand for the early-stage customers who need to develop and optimize their process toward GMP manufacturing.
Per Catalent’s management, the expanded services across multiple biologic therapies will likely offer customers the opportunity to leverage its end-to-end capabilities in these areas and accelerate their programs to the clinic and beyond. This, in turn, is expected to mitigate potential risks and delays by providing planning efficiencies, an integrated contract, streamlined project management support and harmonized quality systems.
Industry Prospects
Per a report by Coherent Market Insights, the global cell and gene therapy market was valued at $15,580.3 million in 2022 and is anticipated to witness a CAGR of 24.7% between 2022 and 2030. Factors like the increasing demand for innovative treatments and the developing interest in cell and gene treatments for cancer treatments are likely to drive the market.
Given the market potential, the latest product expansion will likely provide a significant impetus to Catalent in the cell and gene therapy space worldwide.
Notable Developments
In the same press release, Catalent confirmed recent advancements across the Biologics segment, including high-speed syringe filling line investments at its sites in Anagni, Italy, and Bloomington, IN.
At the same time, the company shared that Catalent Biologics’ European drug product network also recently saw the completion of upgrades to its site in Limoges, France. This makes it the company’s European center of excellence for early-phase clinical and small-scale commercial biologics formulation development and drug product fill/finish.
Price Performance
Shares of Catalent have lost 63.4% in the past year compared with the industry’s 4.4% decline. The S&P 500 has risen 10.3% rise in the said time frame.
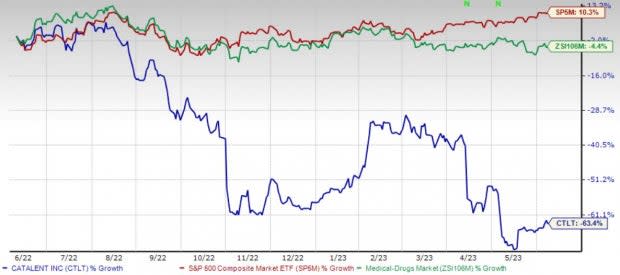
Image Source: Zacks Investment Research
Zacks Rank & Stocks to Consider
Currently, Catalent carries a Zacks Rank #5 (Strong Sell).
Some better-ranked stocks in the broader medical space are Hologic, Inc. HOLX, Merit Medical Systems, Inc. MMSI and Boston Scientific Corporation BSX.
Hologic, carrying a Zacks Rank #2 (Buy) at present, has an estimated growth rate of 5.1% for fiscal 2024. HOLX’s earnings surpassed estimates in all the trailing four quarters, the average being 27.3%. You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Hologic has gained 8.9% compared with the industry’s 6.5% rise in the past year.
Merit Medical, carrying a Zacks Rank #2 at present, has an estimated long-term growth rate of 11%. MMSI’s earnings surpassed estimates in all the trailing four quarters, the average surprise being 20.2%.
Merit Medical has gained 45.5% compared with the industry’s 11.7% rise over the past year.
Boston Scientific, carrying a Zacks Rank #2 at present, has an estimated long-term growth rate of 11.5%. BSX’s earnings surpassed estimates in two of the trailing four quarters and missed in the other two, the average surprise being 1.9%.
Boston Scientific has gained 37.2% against the industry’s 28.8% decline over the past year.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Boston Scientific Corporation (BSX) : Free Stock Analysis Report
Hologic, Inc. (HOLX) : Free Stock Analysis Report
Merit Medical Systems, Inc. (MMSI) : Free Stock Analysis Report
Catalent, Inc. (CTLT) : Free Stock Analysis Report

 Yahoo Finance
Yahoo Finance 