Charles River (CRL) to Create New Gene Therapy for Hemophilia A
Charles River Laboratories International, Inc. CRL recently inked an agreement with ASC Therapeutics, a privately-held biopharmaceutical company, to manufacture a second-generation gene therapy for hemophilia A, ASC618. The ASC618 program has gained IND clearance and other key regulatory designations in the United States and Europe.
Hemophilia A is caused due to a deficiency of the blood clotting factor VIII (FVIII), a protein whose instructions are provided by a gene F8. ASC618 is intended to provide a shortened yet optimized version of the gene to liver cells.
The latest collaboration will leverage Charles River’s end-to-end contract development and manufacturing organization (CDMO) capabilities. It is expected to build on the company’s prior acquisitions of Cognate BioServices, Cobra Biologics and Vigene Biosciences, which expanded its comprehensive cell and gene therapy portfolio to span each major CDMO platforms, i.e., cell therapy, viral vector and plasmid DNA production.
More on the News
ASC Therapeutics has been collaborating with Charles River since 2019, to use the company’s expertise in Good Manufacturing Practice (GMP)-virus manufacturing and established processes for AAV production and purification.
The companies have worked together to develop a high-yield upstream technique for AAV8 production, optimized the downstream purification methods to produce more predictable drug product output and fine-tuned a scalable manufacturing process in upstream and downstream. Per Charles River’s management, the collaboration with ASC Therapeutics has relied on a strong and transparent relationship to enable a seamless transition between process development and GMP production throughout the past three years.
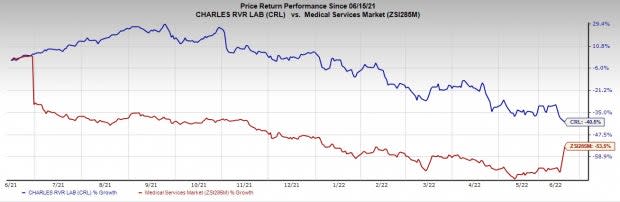
Image Source: Zacks Investment Research
Meanwhile, management at ASC Therapeutics believes that the collaboration with Charles River will likely further their manufacturing knowledge as the company advances to the next phase of therapeutic development.
Industry Prospects
Per a report by MarketsandMarkets, the global gene therapy market is expected to witness a CAGR of 27.8% during 2019 to 2024. Factors such as the increasing incidence of cancer and other target diseases, greater availability of reimbursements and the growing funding for gene therapy research can be attributable to market growth.
Given the market prospects, Charles River’s latest collaboration to manufacture a second-generation gene therapy for hemophilia A, seems reasonable.
Other Notable Developments
In April 2022, Charles River and Valo Health, Inc (“Valo”) launched Logica, an artificial intelligence-powered drug solution. The Logica directly translates clients’ biological insights into optimized preclinical assets. It utilizes Valo’s Opal Computational Platform and Charles River’s preclinical expertise to offer clients transformed drug discovery through a single integrated offering.
The same month, the company acquired San Diego, CA-based contract vivarium research firm ExploraBioLabs Holdings, Inc. The acquisition was settled for approximately $295 million in cash, subject to customary closing adjustments. This buyout complements and fortifies the Charles River Accelerator and Development Lab (“CRADL”) operation within the broader Insourcing Solutions business.
Share Price Performance
The stock has outperformed its industry in the past year. It has declined 40.5% compared with the industry’s 53.5% fall.
Zacks Rank and Key Picks
Currently, Charles River carries a Zacks Rank #3 (Hold).
A few better-ranked stocks in the broader medical space are Alkermes plc ALKS, AMN Healthcare Services, Inc. AMN and Medpace Holdings, Inc. MEDP.
Alkermes has an estimated long-term growth rate of 25.1%. Alkermes’ earnings surpassed estimates in the trailing four quarters, the average surprise being 350.5%. It currently carries a Zacks Rank #1 (Strong Buy). You can see the complete list of today’s Zacks #1 Rank stocks here.
Alkermes has outperformed the industry in the past year. ALKS has gained 11% against the industry’s 46% decline in the said period.
AMN Healthcare has a long-term earnings growth rate of 1.1%. The company surpassed earnings estimates in the trailing four quarters, delivering a surprise of 15.6%, on average. It currently flaunts a Zacks Rank #1.
AMN Healthcare has outperformed its industry in the past year. AMN has gained 0.7% against the industry’s 53.5% fall.
Medpace has a historical growth rate of 27.3%. Medpace’s earnings surpassed estimates in the trailing four quarters, the average surprise being 17.1%. It currently has a Zacks Rank #2 (Buy).
Medpace has outperformed its industry in the past year. MEDP has declined 26% compared with the industry’s 53.5% fall.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
To read this article on Zacks.com click here.

 Yahoo Finance
Yahoo Finance 