Lilly (LLY) COVID-19 Antibody Gets FDA Nod for Use in Kids
Eli Lilly & Company LLY announced that the FDA has expanded the Emergency Use Authorization (EUA) granted to its COVID-19 antibody cocktail, bamlanivimab plus etesevimab to allow its use in high-risk COVID-19 pediatric patients.
With the latest expansion, Lilly’s bamlanivimab and etesevimab can now be given both for the treatment of patients with COVID-19 as well as for post-exposure prophylaxis in high-risk pediatric and infant patients (less than 12 years of age).
Bamlanivimab plus etesevimab was granted EUA by the FDA in February 2021 to treat mild-to-moderate COVID-19 in high-risk patients 12 years of age and older based on data from the BLAZE-1 study. The expansion for pediatric and infant patients was based on the safety and efficacy data of such patients in the BLAZE-1 study.
In September, the FDA expanded the EUA for the cocktail antibody medicine to include the post-exposure prevention (prophylaxis) for the COVID-19 indication. Lilly’s cocktail medicine generated revenues of $217.1 million in the third quarter of 2021.
With the approval in children and infants, Lilly’s bamlanivimab with etesevimab can now be given as treatment and prevention options to high-risk individuals of any age.
So far this year, Lilly’s stock has risen 47.7% compared with an increase of 16.1% for the industry.
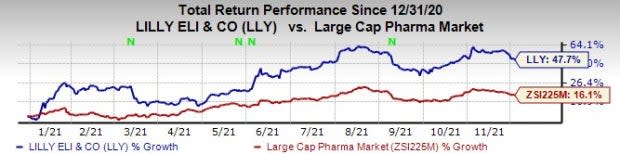
Image Source: Zacks Investment Research
Other monoclonal antibodies approved to treat COVID-19 are Regeneron’s REGN antibody cocktail, REGEN-COV and Glaxo GSK and partner Vir Biotech’s VIR Xevudy (sotrovimab).
REGEN-COV comprises two monoclonal antibodies, casirivimab and imdevimab and has become a significant contributor to its top line in recent quarters. Regeneron’s antibody cocktail, REGEN-COV, generated total sales of $1.2 billion in the third quarter of 2021.
Glaxo and Vir Biotech’s Xevudy was granted emergency approval by the FDA in May 2021 for treating high-risk COVID-19.
Xevudy contributed 3 percentage points of growth to Glaxo’s total Pharmaceutical sales in the third quarter of 2021. Sotrovimab is not yet approved in Europe though Glaxo and VIR Biotech have a joint procurement agreement with the European Commission to supply up to 220,000 doses of sotrovimab, once it is authorized in Europe.
With the risk of infection rising due to the threat posed by the rapidly spreading and highly contagious Omicron variant, the need for re-designed treatments and vaccines has increased. The Omicron variant has ignited fears that the currently available COVID-19 treatments could be less effective against it.
Lilly is working to understand the neutralization activity of the COVID-19 antibody cocktail against Omicron.
Regeneron has said that REGEN-COV could be less effective against Omicron. Further tests are being conducted to understand the impact of using the actual Omicron variant sequence.
Glaxo and Vir Biotech said that pre-clinical data showed that sotrovimab retains activity against key mutations of the Omicron variant.
Lilly currently carries a Zacks Rank #3 (Hold). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Regeneron Pharmaceuticals, Inc. (REGN) : Free Stock Analysis Report
GlaxoSmithKline plc (GSK) : Free Stock Analysis Report
Eli Lilly and Company (LLY) : Free Stock Analysis Report
Vir Biotechnology, Inc. (VIR) : Free Stock Analysis Report
To read this article on Zacks.com click here.
Zacks Investment Research

 Yahoo Finance
Yahoo Finance 