Merck (MRK) Pauses Enrolment in HIV-1 Prevention Studies
Merck MRK announced that it has paused the enrolment of patients in two phase III studies, IMPOWER 22 and IMPOWER 24, which are evaluating investigational oral inhibitor, islatravir (ISL), for pre-exposure prophylaxis (PrEP) in people at a high risk of HIV-1 infection.
The decision to pause enrolment in the IMPOWER 22 and IMPOWER 24 studies was based on the recommendation of the ISL PrEP external data monitoring committee (“eDMC”). Nonetheless, enrolled participants in both the studies will continue to receive the study medicine.
The IMPOWER 22 study is evaluating the safety and efficacy of ISL, administered once-monthly, in comparison to emtricitabine/tenofovir disoproxil fumarate (FTC/TDF), administered once-daily, as PrEP in cisgender women at a high risk of HIV-1 infection. The IMPOWER 24 is evaluating the safety and efficacy of ISL, administered once-monthly, in comparison to FTC/TDF or emtricitabine/tenofovir alafenamide (FTC/TAF), administered once-daily, in cisgender men and transgender women who have sex with men.
Merck is conducting further analyses of these studies. The company is also implementing additional monitoring measures for the study participants, including increasing the frequency of total lymphocyte and CD4+ T-cell assessments.
Merck’s stock has declined 10.2% this year so far against the industry’s 12.4% rise.
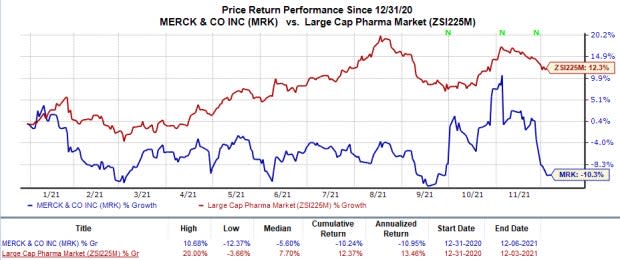
Image Source: Zacks Investment Research
Merck is also evaluating ISL as a treatment for HIV in multiple combinations. Earlier in October, Merck announced top-line data from two phase III studies evaluating an oral fixed-dose combination pill of doravirine/islatravir in two phase III studies in adults with HIV-1 infection. Both the studies achieved their safety and efficacy endpoints.
Last month, Merck announced that it had stopped dosing in the phase II study evaluating the combination of MK-8507 and ISL as a treatment for people living with HIV-1. The dosing was stopped at the recommendation of an eDMC, which observed decreases in total lymphocyte and CD4+ T-cell counts in study participants randomized to receive ISL plus MK-8507.
Merck is also collaborating with Gilead Sciences GILD to evaluate the combination of ISL with the latter’s lenacapavir to develop long-acting HIV treatment options. Earlier in October, Gilead and Merck initiated a phase II study evaluating the combination of a once-weekly treatment regimen of ISL and lenacapavir in people living with HIV who are virologically suppressed on antiretroviral therapy.
Gilead Sciences is a dominant player in the HIV market. Gilead recorded $4.2 billion from HIV product sales for the third quarter of 2021.
Merck faces stiff competition from GlaxoSmithKline GSK, which has developed its own PrEP candidate for HIV, cabotegravir. Glaxo has already submitted a regulatory application to the FDA seeking approval for cabotegravir for PrEP and expects to receive a decision in January 2022.
In January 2021, GlaxoSmithKline announced that the FDA approved a long-acting injectable regimen of cabotegravir and Edurant (rilpivirine) for the treatment of HIV-1 infection in virologically suppressed adults. The combination is being marketed under the trade name Cabenuva.
Merck & Co., Inc. Price
Merck & Co., Inc. price | Merck & Co., Inc. Quote
Zacks Rank & Stock to Consider
Merck currently carries a Zacks Rank #3 (Hold). A better-ranked stock in the overall healthcare sector is Endo International ENDP, which carries a Zacks Rank #1 (Strong Buy) at present. You can see the complete list of today’s Zacks #1 Rank stocks here.
Endo International’s earnings per share estimates for 2021 have increased from $2.64 to $2.84 in the past 30 days. The same for 2022 has increased from $2.44 to $2.47 in the past 30 days.
Earnings of Endo International beat estimates in all the last four quarters, with the average being 57.7%.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
GlaxoSmithKline plc (GSK) : Free Stock Analysis Report
Endo International plc (ENDP) : Free Stock Analysis Report
Merck & Co., Inc. (MRK) : Free Stock Analysis Report
Gilead Sciences, Inc. (GILD) : Free Stock Analysis Report
To read this article on Zacks.com click here.

 Yahoo Finance
Yahoo Finance 