Regeneron (REGN) Q1 Earnings, Sales Miss on Lower Eylea Sales
Regeneron Pharmaceuticals, Inc. REGN delivered lower-than-expected first-quarter 2024 results due to declining sales of its lead drug, Eylea (aflibercept). The company reported earnings per share (EPS) of $9.55, which missed the Zacks Consensus Estimate of $10.20. The company recorded EPS of $10.09 in the year-ago period.
Higher expenses and lower revenues adversely impacted the bottom line.
Total revenues declined 1% year over year to $3.14 billion, which also missed the Zacks Consensus Estimate of $3.19 billion.
Shares are trading down in response to the dismal results.
Regeneron’s shares have risen 2.9% year to date against the industry’s decline of 8.8%.
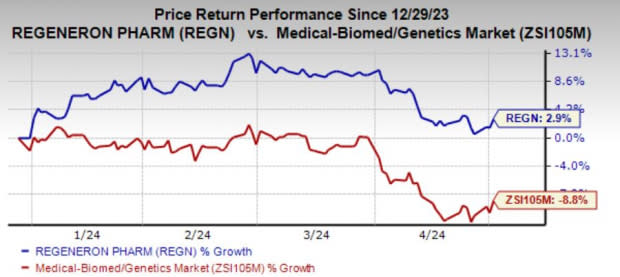
Image Source: Zacks Investment Research
Quarterly Highlights
Eylea’s sales in the United States declined 16% to $1.2 billion, primarily due to increased competition resulting in lower volumes and a reduced net selling price.
Please note that Regeneron co-developed Eylea with the HealthCare unit of Bayer AG BAYRY. Regeneron records net product sales of Eylea in the United States and Bayer does the same outside the country. Regeneron records its share of profits/losses in connection with the sales of Eylea outside the United States.
In August 2023, the FDA approved Eylea HD (higher dose of Eylea) for the treatment of patients with wet age-related macular degeneration, diabetic macular edema and diabetic retinopathy.
Eylea HD generated revenues of $200 million in the United States.
Total revenues include collaboration revenues of $1.27 billion from Sanofi SNY and Bayer. The figure decreased 8.1% from that recorded in the year-ago quarter. Total collaboration revenues missed the Zacks Consensus Estimate of $1.32 billion.
Sanofi’s collaboration revenues increased 14% to $910 million, driven by profits associated with higher Dupixent sales. We note that Sanofi records global net product sales of Dupixent and Kevzara, while Regeneron records its share of profits/losses in connection with global sales of both drugs. While Dupixent’s sales increased 24% year over year to $3.08 billion, the figure marginally missed the Zacks Consensus Estimate of $3.09 billion.
Bayer’s collaboration revenues totaled $356 million, flat year over year.
Regeneron records net product sales of Praluent in the United States and Sanofi does the same outside the country. SNY pays REGN a royalty on such sales. Effective Jul 1, 2022, Regeneron records global net product sales of Libtayo outside the United States and pays a royalty to Sanofi on such sales.
Total Libtayo sales came in at $263.9 million. Praluent’s net sales in the United States were $70 million. Kevzara recorded global sales of $94.1 million, up 20% from the year-ago quarter’s level.
REGEN-COV, its antibody cocktail for COVID-19, generated $1.2 million in sales outside of the United States, down 100% year over year.
Adjusted R&D expenses jumped 16.8% to $1.12 billion due to the advancement of the company's late-stage oncology programs, and higher headcount and headcount-related costs. Adjusted SG&A expenses increased 13.5% to $584 million due to higher commercialization-related expenses to support the launch of Eylea HD and higher headcount and headcount-related costs.
Pipeline and Regulatory Update
The FDA accepted the supplemental biologics license application (sBLA) for Dupixent as an add-on maintenance treatment in adult patients with uncontrolled chronic obstructive pulmonary disease and evidence of type 2 inflammation. The sBLA was granted Priority Review. A regulatory application is also under review in the European Union and Japan.
Regeneron suffered a setback when the FDA issued Complete Response Letters (CRLs) for the BLA for odronextamab, a bispecific antibody targeting CD20 and CD3, in R/R follicular lymphoma (FL) and R/R diffuse large B-cell lymphoma (DLBCL). The enrolment status of the confirmatory trials only was the reason cited in the letters. The CRLs (one for R/R FL and one for R/R DLBCL) did not identify any approval issues with the clinical efficacy or safety, trial design, labeling, or manufacturing. A regulatory application for R/R DLBCL and R/R FL remains under review in the EU.
The FDA accepted the BLA seeking accelerated approval for linvoseltamab, a bispecific antibody targeting BCMA and CD3, to treat adult patients with relapsed/refractory (R/R) multiple myeloma that has progressed after at least three prior therapies. This BLA was also granted priority review with a target action date of Aug 22, 2024.
Regeneron Pharmaceuticals, Inc. Price, Consensus and EPS Surprise
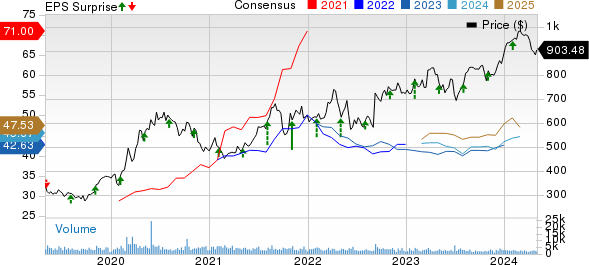
Regeneron Pharmaceuticals, Inc. price-consensus-eps-surprise-chart | Regeneron Pharmaceuticals, Inc. Quote
Other Updates
In April 2024, REGN acquired full development and commercialization rights to 2seventy bio, Inc.'s TSVT oncology and autoimmune preclinical and clinical stage cell therapy pipeline. In exchange, REGN made a $5 million upfront payment, and assumed ongoing program, infrastructure, and personnel costs related to the product candidates acquired.
Regeneron will also pay 2seventy bio a regulatory milestone upon the first major market approval of the first approved product, and, with respect to any approved product, a low single-digit percent royalty on sales.
Our Take
Regeneron’s top and bottom lines missed estimates in the reported quarter. Eylea sales have been under pressure due to competition from Roche’s Vabysmo.
To counter the decline in Eylea sales, Regeneron developed a higher dose of the drug. The initial uptake of Eylea HD is encouraging as Eylea patients transition to the higher dose. However, it may be a while before the higher dose can compensate for the loss in Eylea sales.
Zacks Rank
Regeneron currently carries a Zacks Rank #3 (Hold). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Regeneron Pharmaceuticals, Inc. (REGN) : Free Stock Analysis Report
Sanofi (SNY) : Free Stock Analysis Report
Bayer Aktiengesellschaft (BAYRY) : Free Stock Analysis Report
2seventy bio, Inc. (TSVT) : Free Stock Analysis Report

 Yahoo Finance
Yahoo Finance 